Preparation method of pemetrexed or pemetrexed salt
A technology for pemetrexed and salt formation, applied in the field of preparation of pemetrexed or its salt, can solve the problems of few reaction steps, poor product purity, low yield and the like, achieve high product yield and reduce production cost , the effect of short reaction routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a constant temperature water bath at 35°C, stir 100ml of deionized water, 10.0g (68.0mmol) of L-glutamic acid, and 7.0g (69.0mmol) of NMM (N-methylmorpholine) until completely dissolved, then add 300ml of N-methyl pyrrolidone (NMP) to give a clear solution.
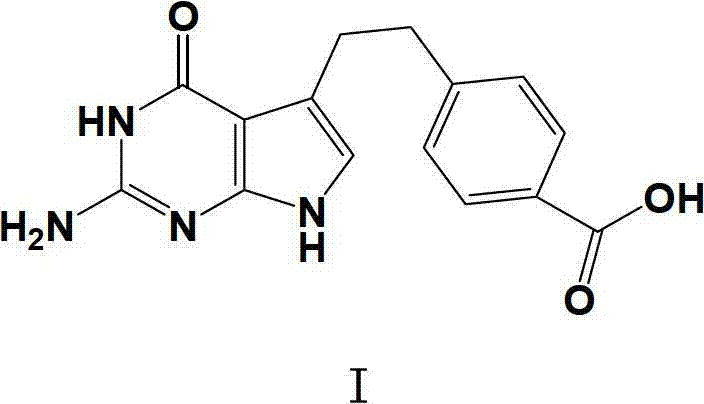
[0020] Under nitrogen protection, 7.0g (69.0mmol) NMM and 11.7g (66.6mmol) 4-chloro-2,6-dimethoxytriazine (CDMT) were added to 4-[2-(2-amino-4 , 7-dihydro-4-oxo-3H-pyrrolo (2,3-d) pyrimidin-5-yl) ethyl] benzoic acid (10g, 33.5mmol) in NMP (150ml) solution, constant temperature water bath 35 Stir for 1 hour at °C. Slowly drop this reaction solution into the above-prepared L-glutamate solution within about 0.5 hours to obtain a clear reaction solution, stir in a constant temperature water bath at 35°C for 1 hour, filter with suction, and adjust the pH of the filtrate to 3-4 with 2N hydrochloric acid , transfer the reaction solution into a 5L reaction bottle, slowly add 4L deionized water, stir in a constant temp...
Embodiment 2
[0023] The method is the same as in Example 1, the difference is that the volume of NMP added when preparing the L-glutamate solution is 200ml, and finally 12.5g of a light green solid is obtained, and 4-[2-(2-amino-4,7-dihydro -4-oxo-3H-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl]benzoic acid, the yield was 87.4%.
[0024] 1H NMR (DMSO-d6) σ12.30 (2H, s), 10.55 (1H, s), 10.11 (1H, s), 8.42 (1H, d, J=7.7Hz), 7.78 (2H, d, J=7.9 Hz), 7.25 (2H, d, J=7.9Hz), 6.30 (1H, s), 5.97 (2H, s), 4.40 (1H, m), 2.98 (2H, t, J=7.0Hz), 2.86 ( 2H, t, J=7.0Hz), 2.35 (2H, t, J=7.5Hz), 2.13-2.06 (1H, m), 2.00-1.95 (1H, m).
Embodiment 3
[0026] The method is the same as in Example 1, the difference is that the volume of NMP added when preparing the L-glutamate solution is 100ml, and finally 12.2g of a light green solid is obtained, and 4-[2-(2-amino-4,7-dihydro -4-oxo-3H-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl]benzoic acid, the yield was 85.3%.
[0027] 1H NMR (DMSO-d6) σ12.31 (2H, s), 10.54 (1H, s), 10.12 (1H, s), 8.40 (1H, d, J=7.5Hz), 7.78 (2H, d, J=8.0 Hz), 7.27 (2H, d, J=8.0Hz), 6.31 (1H, s), 5.97 (2H, s), 4.42 (1H, m), 2.98 (2H, t, J=7.1Hz), 2.86 ( 2H,t,J=7.1Hz), 2.35(2H,t,J=7.5Hz), 2.10-2.05(1H,m), 2.00-1.96(1H,m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com