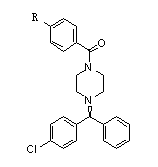
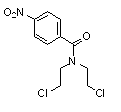
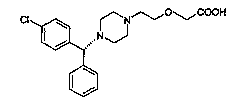
New method for synthesizing levocetirizine and key intermediate thereof
A technology of levocetirizine and compound, applied in the field of new intermediates, key intermediates for synthesizing levocetirizine, can solve the problems of unsatisfactory purity and yield of levocetirizine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1, the preparation of N,N-dihydroxyethyl p-nitrobenzamide
[0068] Add 75g of dichloromethane, 30g of sodium carbonate, 12g of diethanolamine, and 3g of sodium sulfate into the reaction vessel, cool down to -5-0°C while stirring, and then slowly add 15g of p-nitrobenzoyl chloride. React for 2.5 hours, filter, add 100 g of water to dissolve the filter cake, and extract twice with 40 g of dichloromethane; combine the organic phases, add sodium sulfate to dry, filter and concentrate under reduced pressure to obtain the title compound.
Embodiment 2
[0069] Embodiment 2, the preparation of N, N-bis (2-chloroethyl) p-nitrobenzamide
[0070] Add 90 g of dichloromethane to the product obtained in Example 1, lower the temperature to -5-0° C. under stirring, add thionyl chloride dropwise, and react for 1 hour after the drop is complete, then add 250 g of 10% sodium bicarbonate solution, and divide The organic phase was taken, washed with 20 g of saturated brine, dried, filtered and concentrated under reduced pressure to obtain the title compound.
Embodiment 3
[0071] Embodiment 3, the preparation of (-)-1-[(4-chlorophenyl) benzyl]-4-p-nitrobenzoylpiperazine
[0072] Put 20 g of the product obtained in Example 2 into a reaction vessel, add 10 g of diisopropylethylamine and 1 g of sodium iodide, then stir the mixture and raise the temperature to 100-110 ° C, keep it warm for 2 hours, and add R (-)-4 chlorobenzhydrylamine 10g, keep warm for 10 hours after dripping, concentrate under reduced pressure, recover diisopropylethylamine, then add methanol 45g, reflux reaction at about 65°C for 3 hours, then cool to 5 -10°C, filter, wash the filter cake with methanol first, then wash with water, and dry to obtain the title compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com