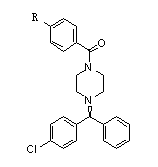
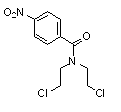
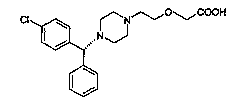
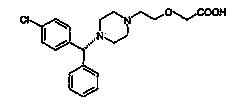
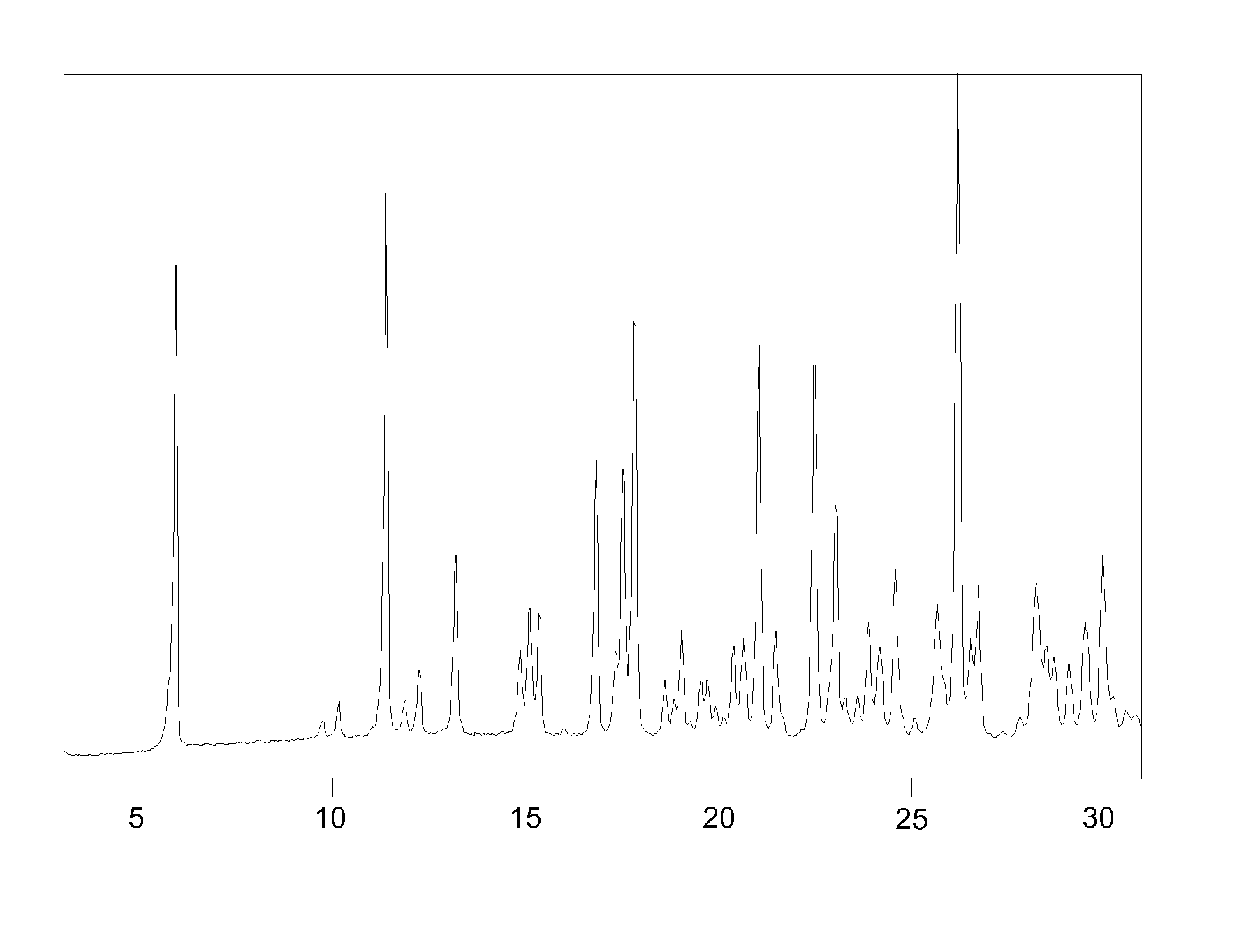
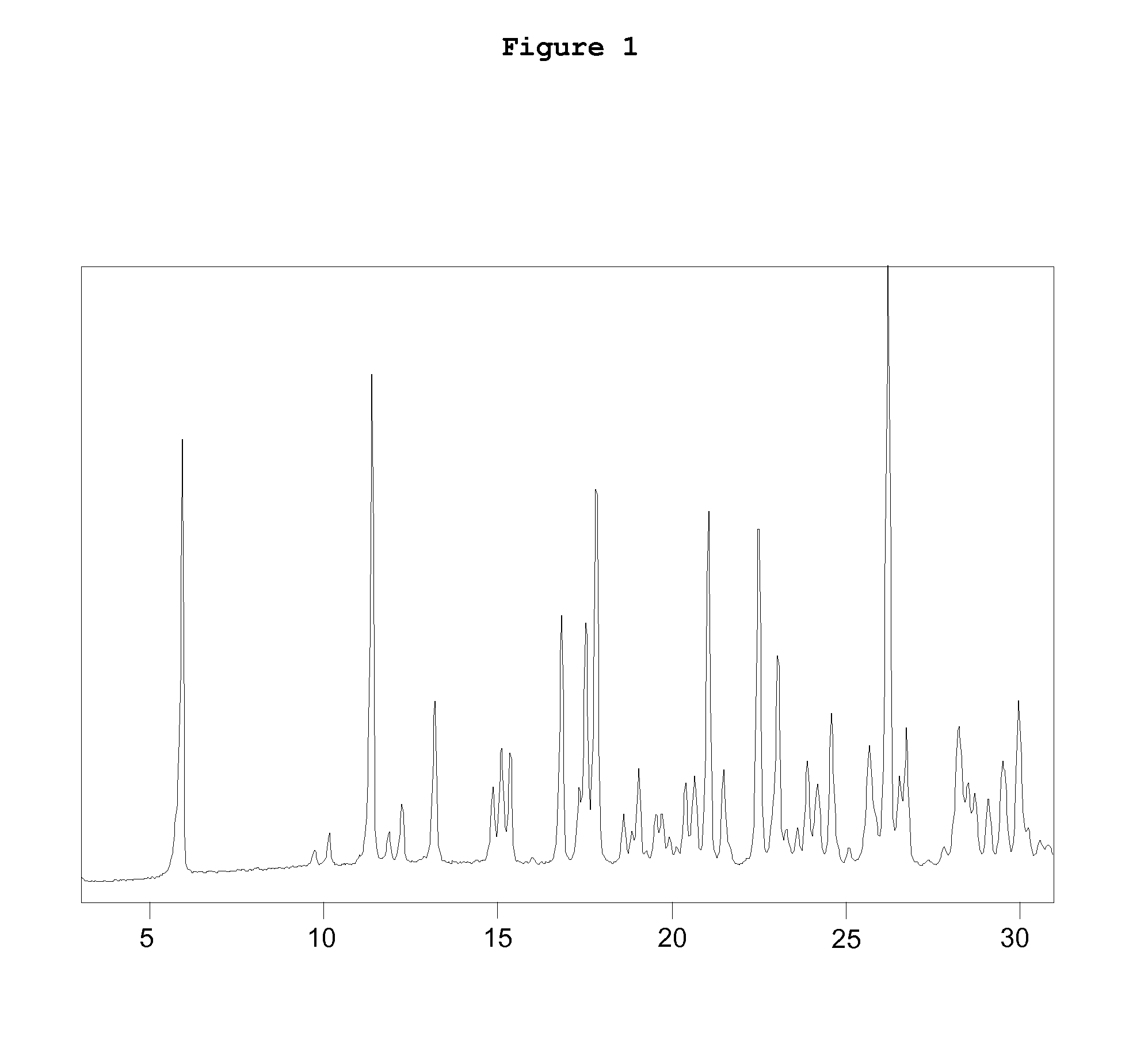
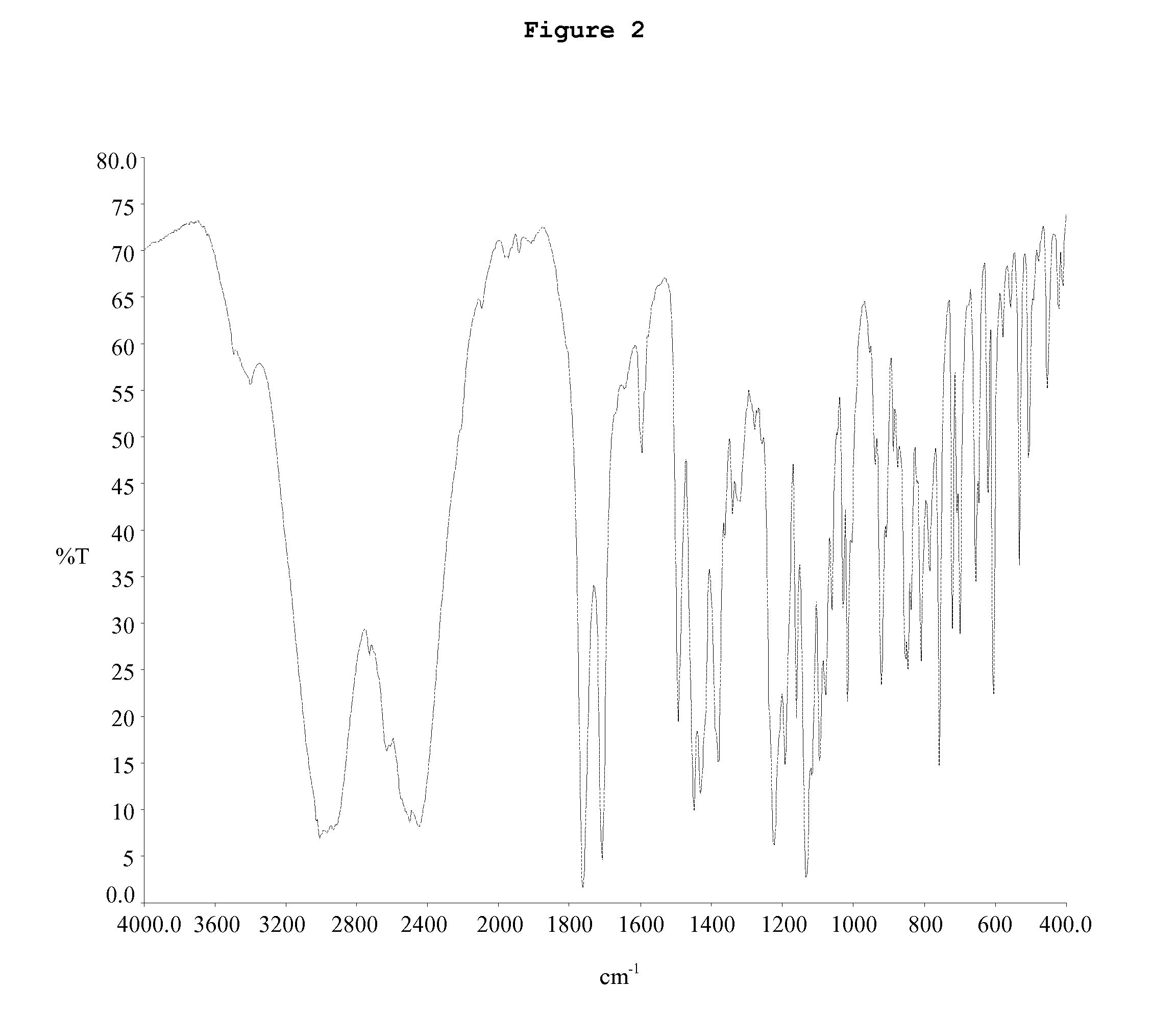
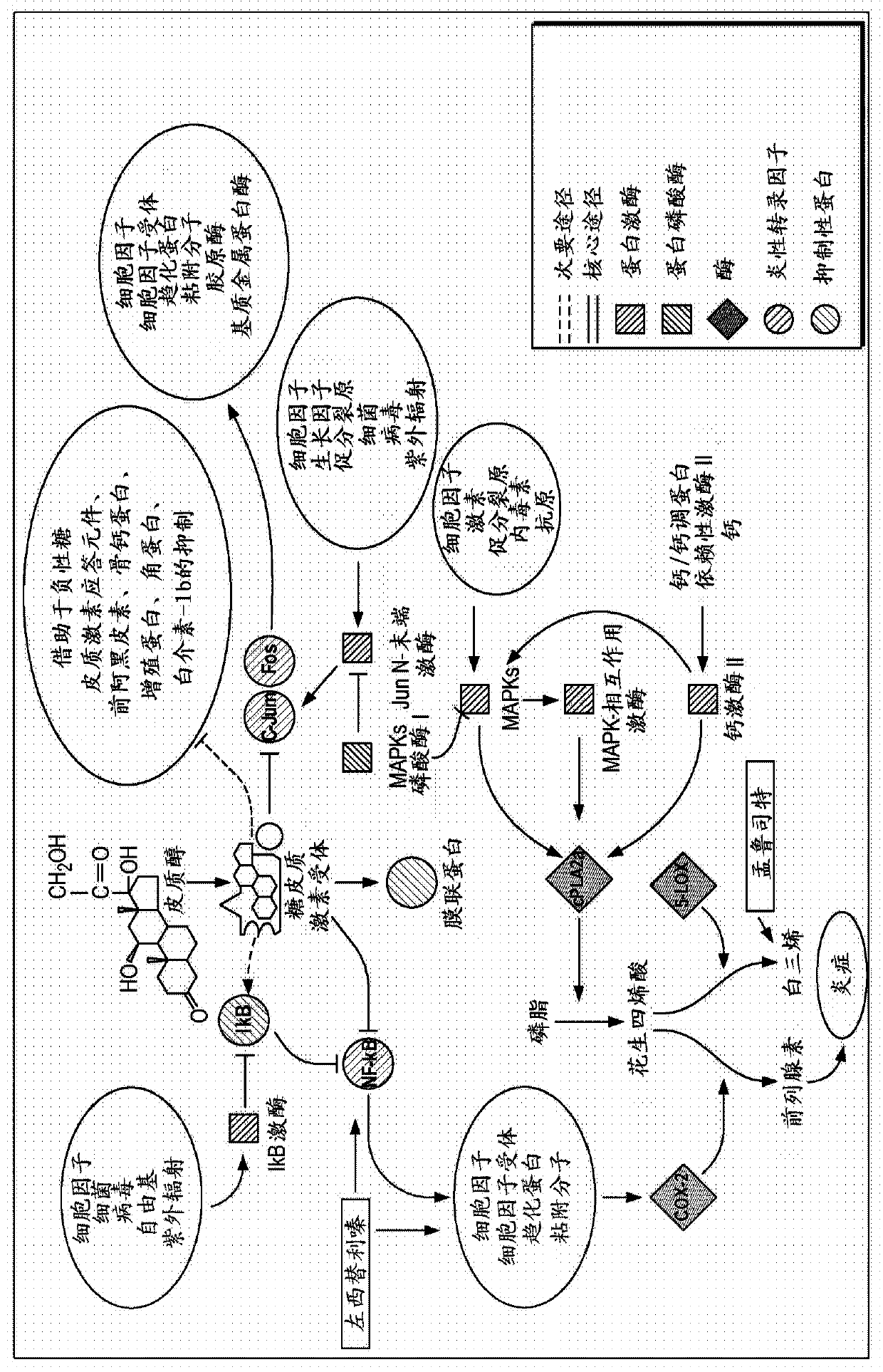
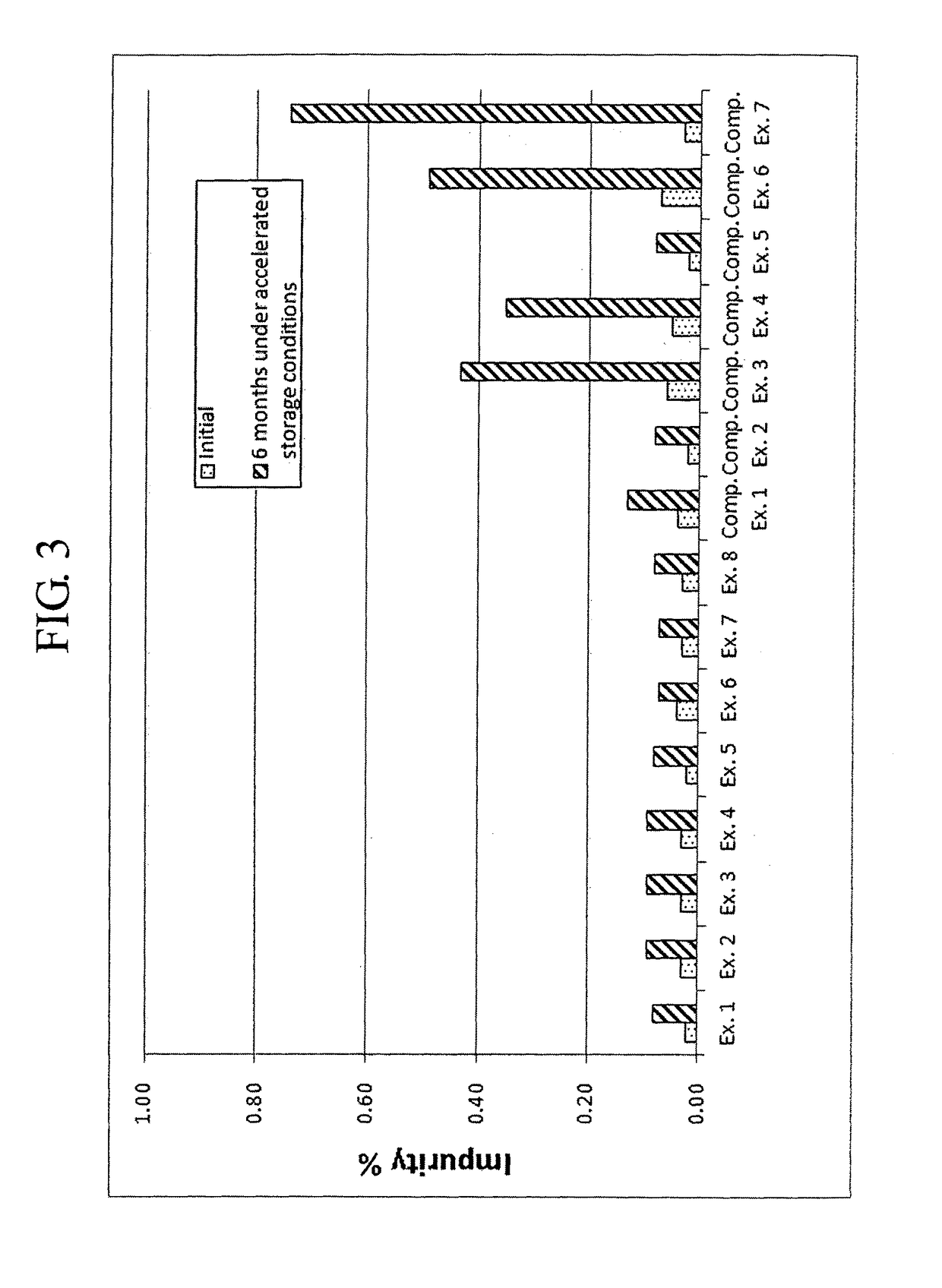
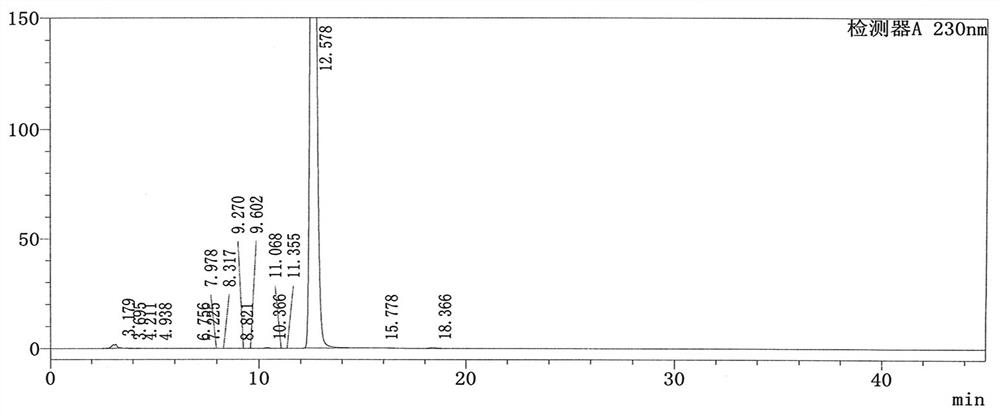
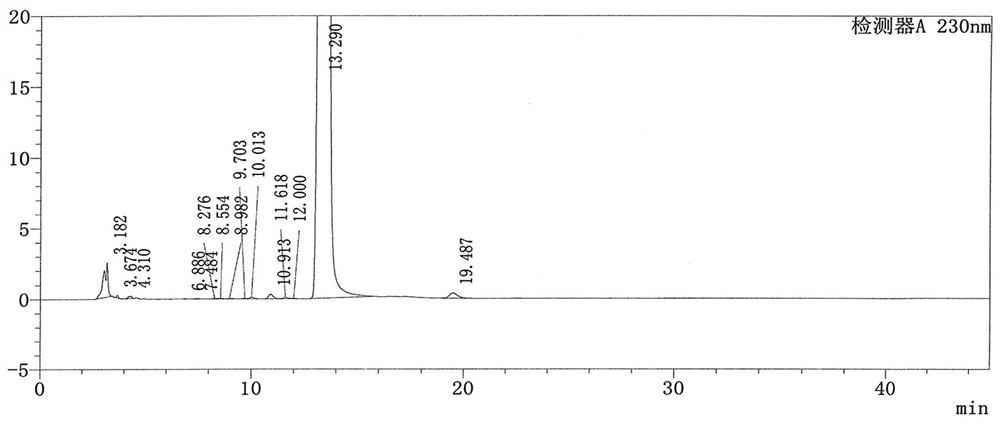
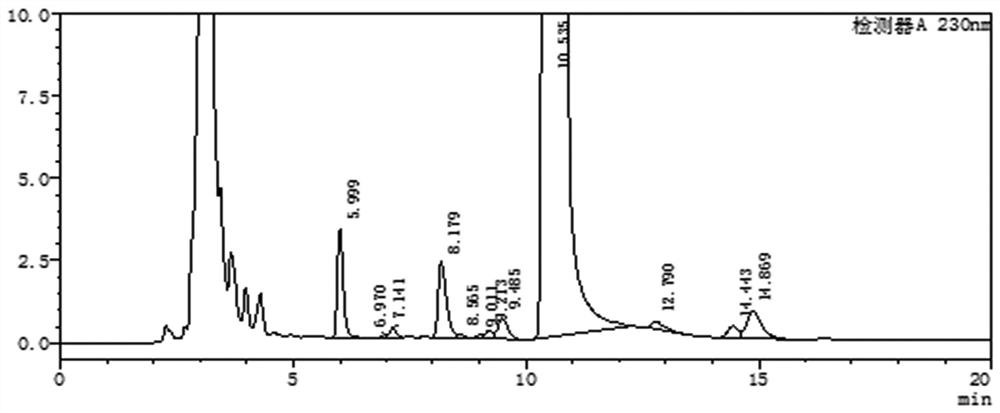
Patents
Literature
30 results about "Levocetrizine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
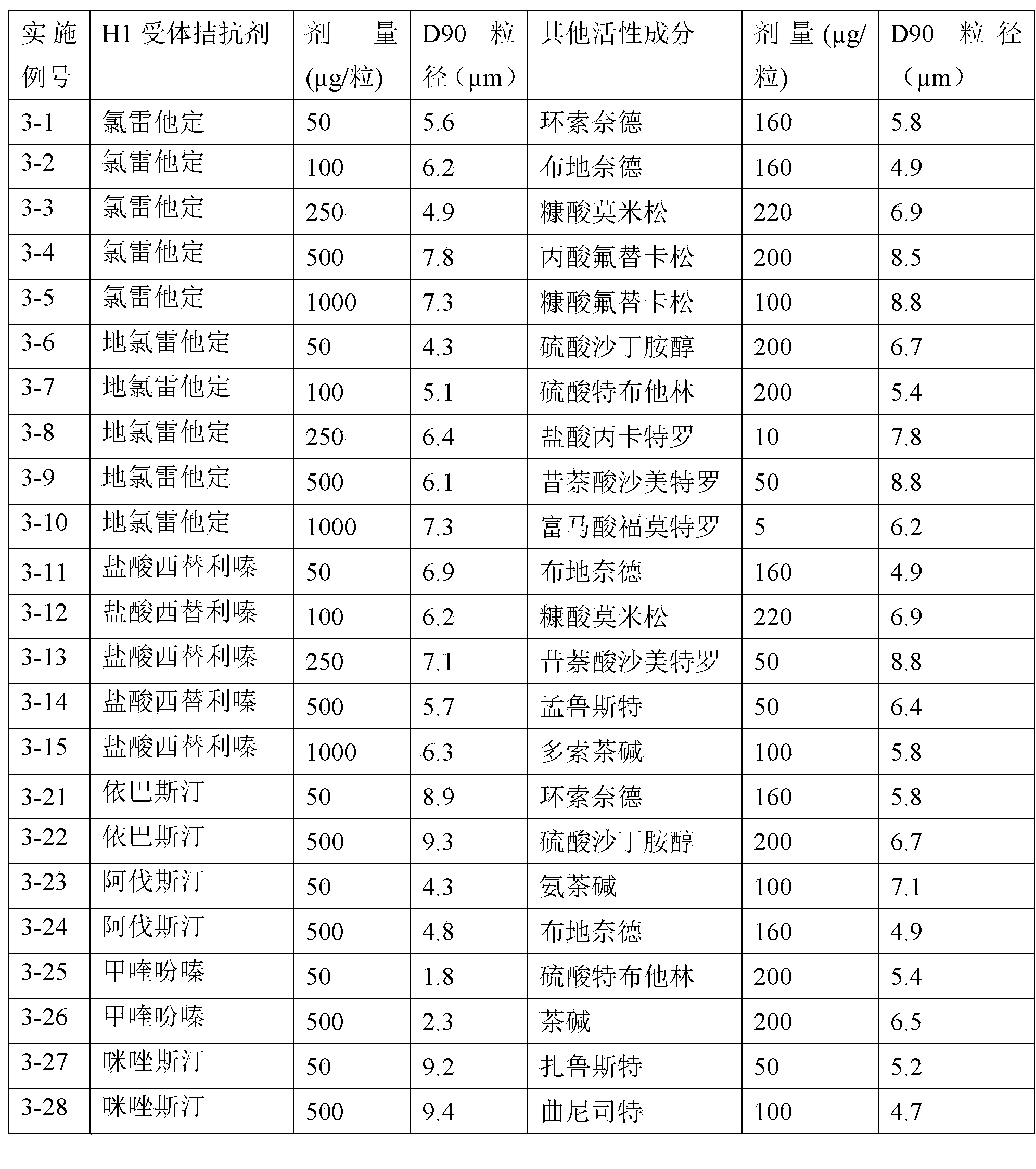
Solution agent of antiallergi medicine contg. levocetirizine
InactiveCN1418630AComfortable tasteEasy to acceptOrganic active ingredientsImmunological disordersDrugLevocetirizine
The present invention provides an antianaphylactic medicine solution preparation containing Levocetirizine. In 1000 ml of medicine composite solution 0.5-20g of zocetirizine or its pharmacal acceptable salt, 20-100 g of polyvinyl pyrrolidone, 5-50g of poloxamer and 10-250g of polyethylene glycol 400 are contained.
Owner:CHONGQING HUAPONT PHARMA
Levocetirizine dihydrochloride granule and preparation and detection methods thereof
ActiveCN101669913ALower control costsSimple processOrganic active ingredientsPharmaceutical non-active ingredientsCurative effectLactose
The invention discloses a levocetirizine dihydrochloride granule and preparation and detection methods thereof. The specification of the levocetirizine dihydrochloride particle is 2.5 mg, and milk sugar is used as filling agent. The levocetirizine dihydrochloride granule dispenses with the process of disintegration of tablets and capsules in a human body, the degree of dispersion in the human bodyis superior to the tablets and the capsules, and the absorption is faster than the tablets, the capsules and dispersible tablets; and the flowability, the dispersibility and the adhesiveness are better than the dispersible tablets, the granule is convenient to take, the mouthfeel is easier to adjust, and the curative effect of the medicine can be guaranteed to be better played.
Owner:HAINAN HONZ PHARMA
Levo-cetirizine hydrochloride orally disintegrating tablets and preparation method thereof
InactiveCN101310711AFast disintegrationFast absorptionOrganic active ingredientsPill deliveryCetirizine HydrochlorideTreatment effect
The invention relates to a hydrochloric acid levocetirizine orally disintegrating tablet and a preparation method thereof. The hydrochloric acid levocetirizine orally disintegrating tablet is obtained by directly pressing principal medicine and accessories. The tablet of the invention can be dissolved fast in a mouth cavity and has no grit feeling and high biological availability, which takes effect fast and can have curative effect faster.
Owner:海南高升医药科技开发股份有限公司
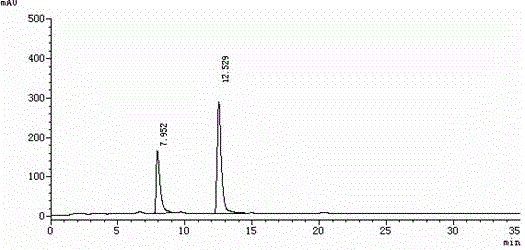
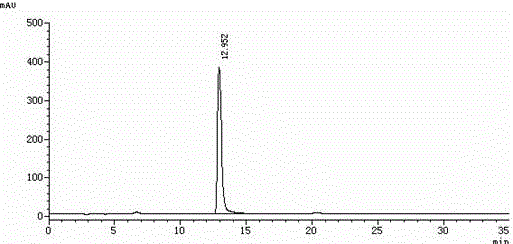
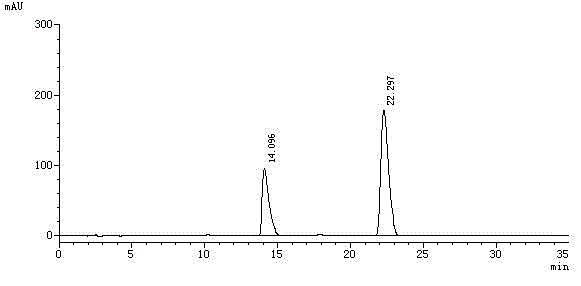
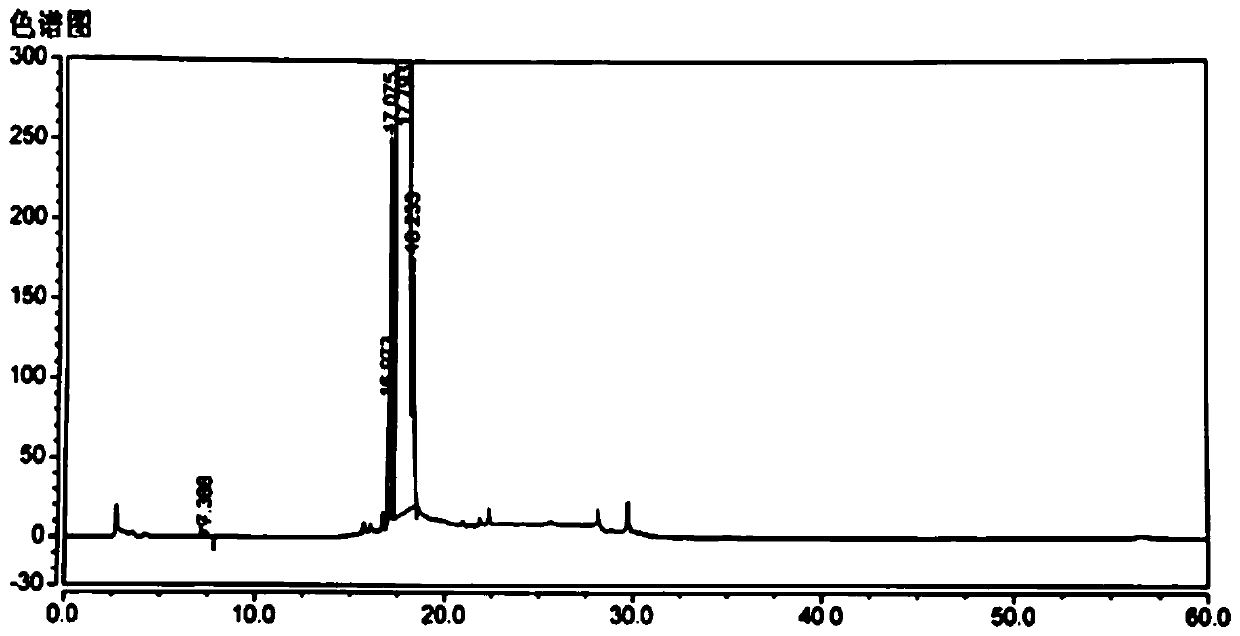
Method for separating and measuring levocetirizine dihydrochloride and related substances by using high performance liquid chromatography
ActiveCN110726788AEasy to separateStrong specificityComponent separationPolyethylene glycolHydrochloric acid
The invention relates to a method for separating and measuring levocetirizine dihydrochloride and 5 related substances by using high performance liquid chromatography. According to the method, a chromatographic column is a C18 chromatographic column, detection is performed after the elution of a mobile phase containing a mixed solution of sodium heptanesulfonate and acetonitrile, and alkali is added to the mobile phase. By adopting the method, separation and detection can be simultaneously performed any one or more of 5 related substances in a levocetirizine dihydrochloride tablet, that is, levocetirizine dihydrochloride, levocetirizine lactose ester thereof, levocetirizine polyethylene glycol ester, p-chlorobenzophenone, p-chlorobenzyl alcohol and p-chlorodiphenylmethylpiperazine. Compared with the prior art, the degree of separation of the levoctirizine hydrochloride and the related substances is good, the sensitivity is high and the separation and detection efficiency is improved.
Owner:HUNAN JIUDIAN PHARMA
H1-receptor-antagonist-containing inhalation preparation
The invention relates to an H1-receptor-antagonist-containing inhalation preparation which contains an H1 receptor antagonist and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
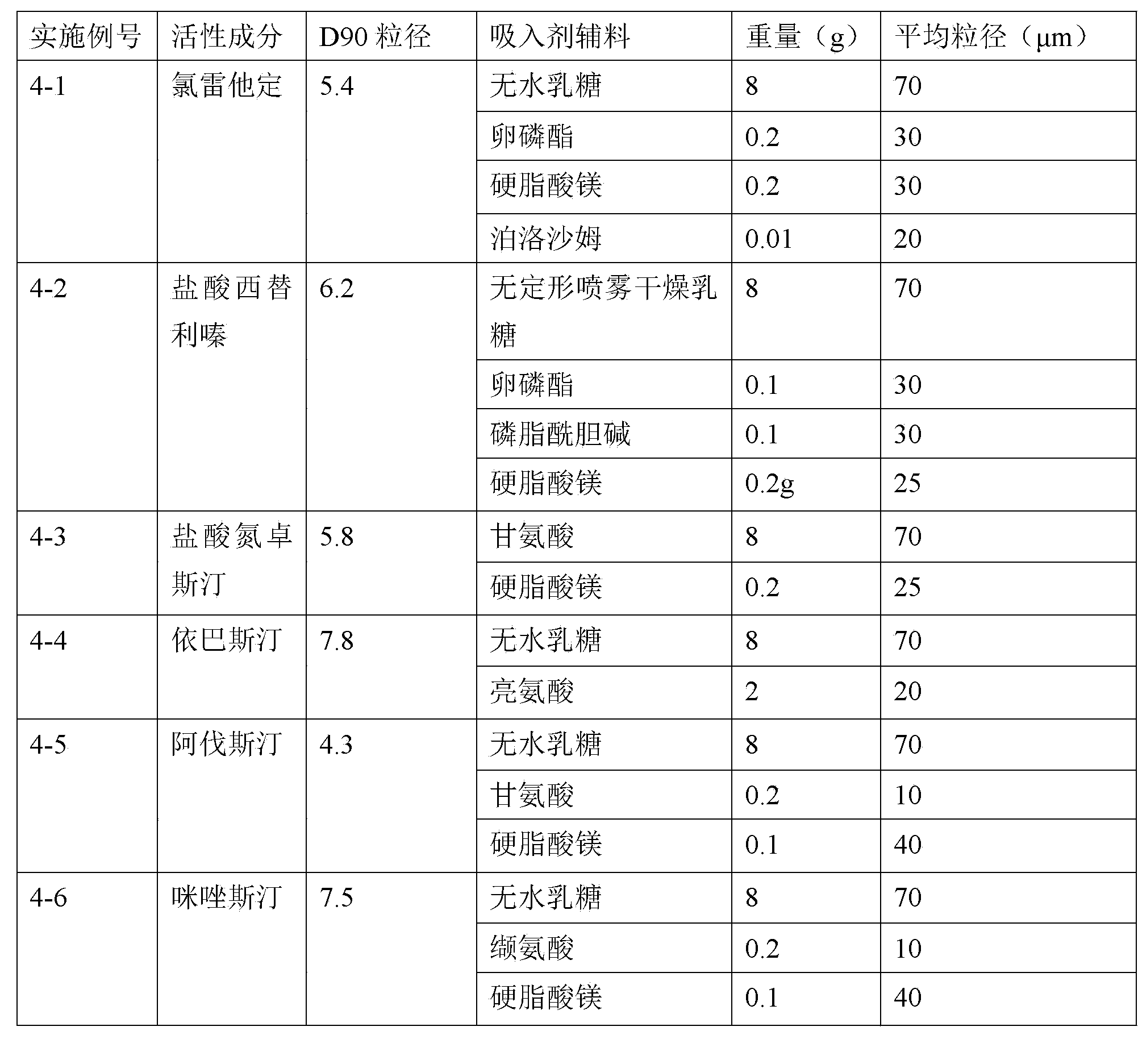
Method for testing related substances of levocetirizine hydrochloride intermediate
The invention belongs to the field of analytical chemistry, and discloses a method for separately testing a levocetirizine hydrochloride intermediate and related substances of the levocetirizine hydrochloride intermediate by liquid chromatography. The method comprises the following steps: a chromatographic column taking octadecylsilane chemically bonded silica as a filler uses a certain proportion of buffer salt solution-organic phase as a mobile phase to quantificationally test the content of the levocetirizine hydrochloride intermediate and the content of the related substances of levocetirizine hydrochloride intermediate, so that the quality of the levocetirizine hydrochloride intermediate is effectively controlled, and the controllable quality of levocetirizine hydrochloride is ensured. The method is high in specificity and accuracy, and simple and convenient to operate.
Owner:AVENTIS PHARMA HAINAN
Levocetirizine hydrochloride syrup and preparation method
InactiveCN103860462AComfortable tasteEasy to acceptOrganic active ingredientsPharmaceutical delivery mechanismDrugs preparationsBitter taste
The invention belongs to the field of medicinal preparations and discloses levocetirizine hydrochloride syrup and a preparation method. The levocetirizine hydrochloride syrup and the preparation method have the advantages that the bitter taste of the levocetirizine hydrochloride is covered, the adaptability of clinical medicines is improved, the treating effect is more ideal, and the preparation process is simple and is high in reliability.
Owner:AVENTIS PHARMA HAINAN
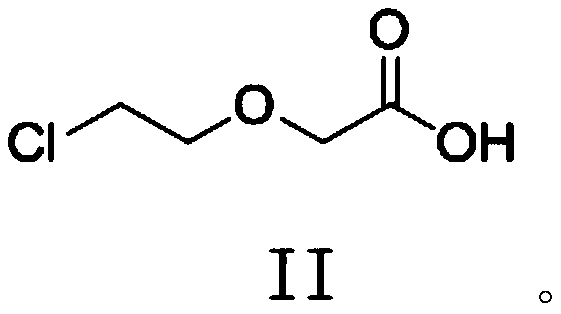
New method for synthesizing levocetirizine and key intermediate thereof
InactiveCN103044356AOrganic compound preparationCarboxylic acid amides preparationChlorobenzeneCombinatorial chemistry
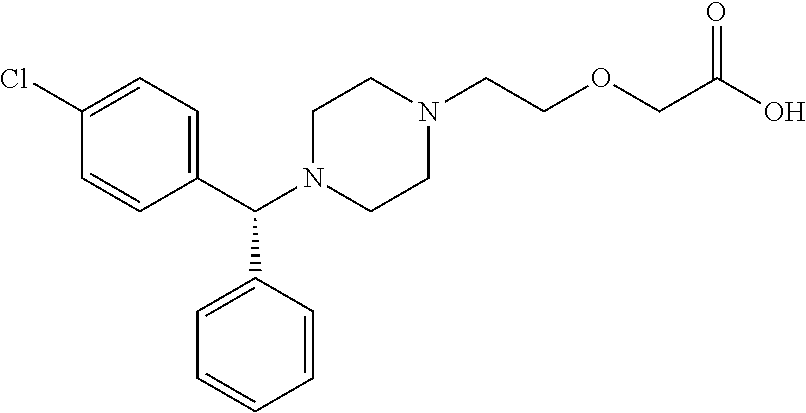
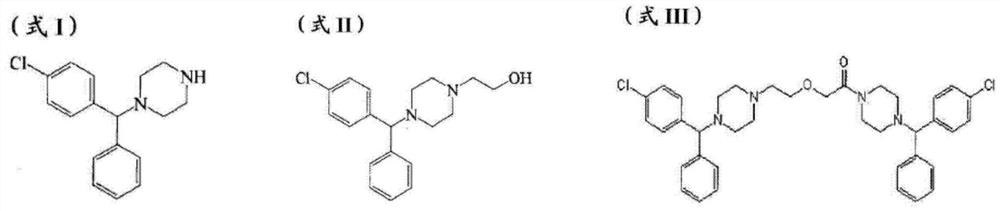
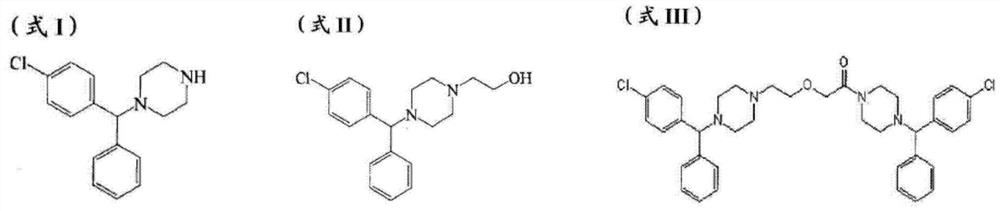
The invention provides a new compound, namely a compound as shown in formula (IV), wherein R refers to Cl, Br, NO2, OH and OR ', and R' refers to alkyl. The invention further provides application of the new compound in synthesis of levocetirizine, including application in synthesis for preparing (-)-1-((4-chlorphenyl)benzyl) piperazine, as well as application as an intermediate for synthesis of the levocetirizine. The invention further provides compounds (II) and (III) which can be used in the synthesis process of the compound (IV).
Owner:HUNAN JIUDIAN PHARMA
Novel synthetic process for levocetirizine and key intermediates
The invention provides a novel compound, namely, the compound in formula (IV), wherein, R is selected from Cl, Br, NO2, OH, tosyl, OR' or alkyl, and R' is alkyl. The invention provides applications of the compound in synthesis of levocetirizine, comprises an application of the compound in synthesis of (-)-1-[(4-chlorophenyl) benzyl] piperazine, and provides intermediates which can be used for synthesis of levocetirizine. The invention further provides compounds (II) and (III) which can be used for synthetic process for the compound (IV).
Owner:HUNAN JIUDIAN PHARMA
Compound pharmaceutical composition for motion sickness
InactiveCN105193805AGreat tasteImprove medication complianceDigestive systemHeterocyclic compound active ingredientsMotion sicknessChlorobenzene
The invention discloses a compound pharmaceutical composition for motion sickness. The compound pharmaceutical composition comprises scopolamine and antihistamine type drugs, wherein chlorpheniramine, dimenhydrinate and levocetirizine are selected as the antihistamine type drugs preferably. Dosage forms suitable for the adult, the old and children can be prepared from the compound pharmaceutical composition and auxiliary materials acceptable in pharmaceutical preparation.
Owner:天津市聚星康华医药科技有限公司
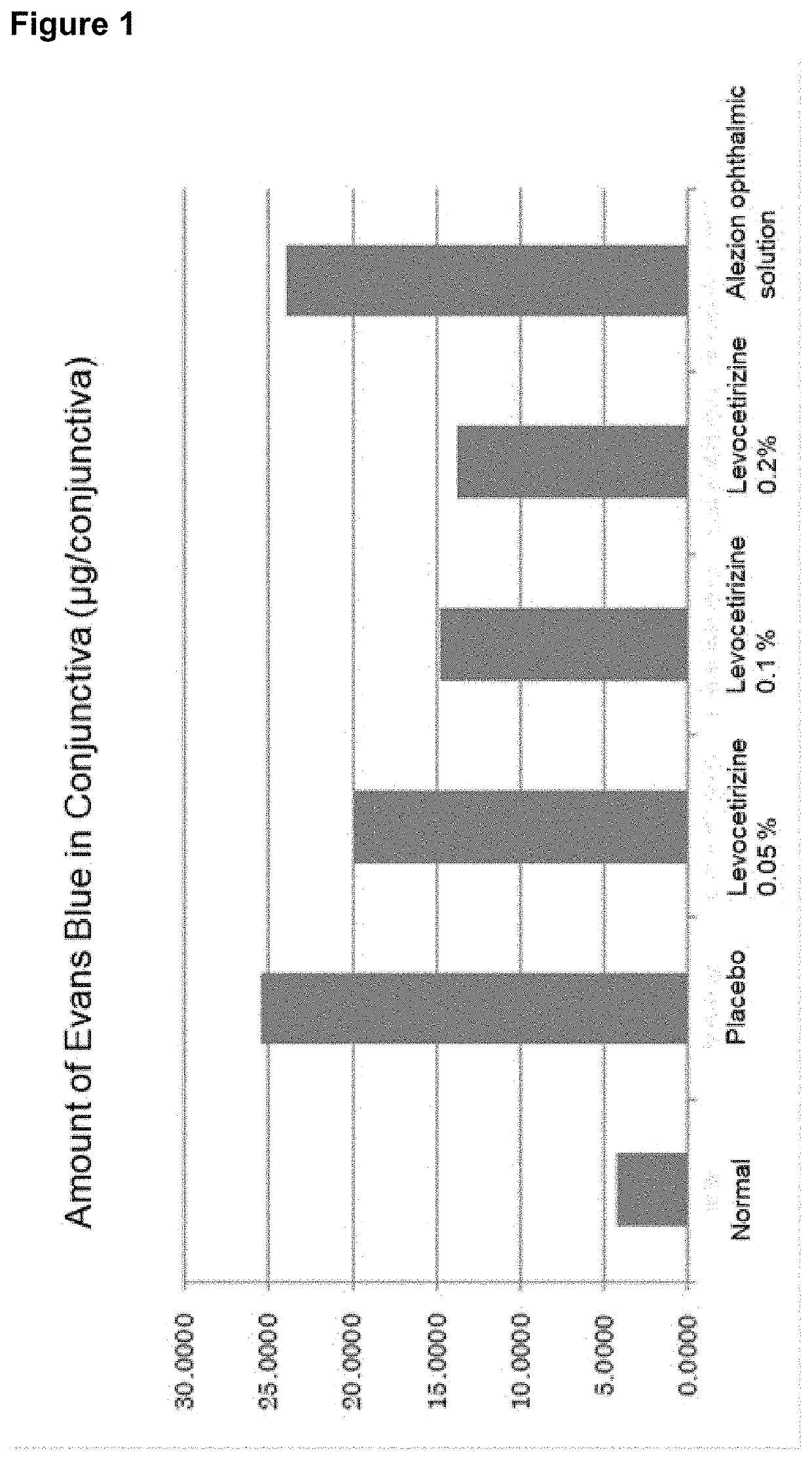
Aqueous composition for eye drops and nasal drops
ActiveUS20200030320A1Process safety and stabilityUseful in treatmentOrganic active ingredientsSenses disorderOrganic acidActive agent
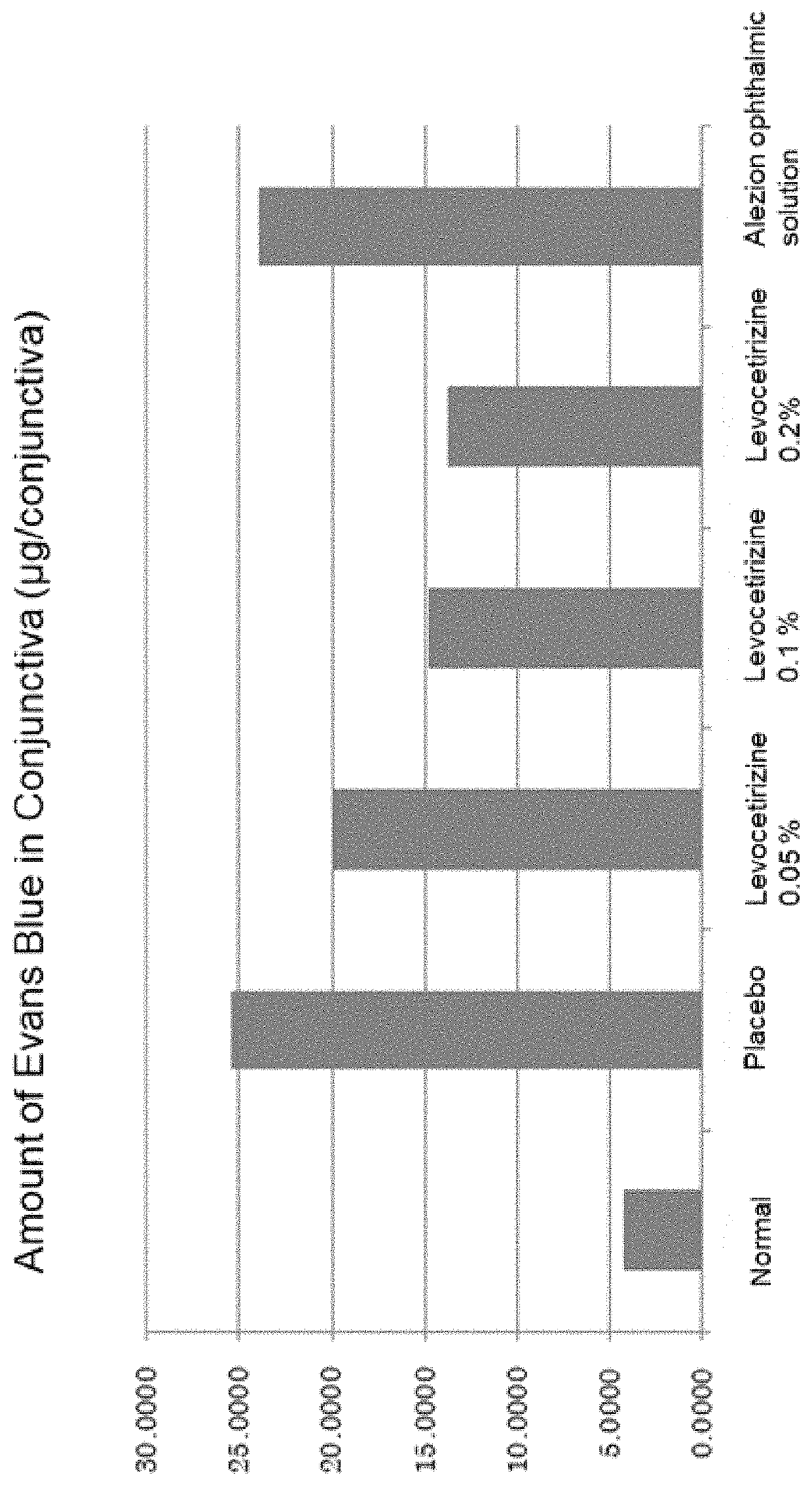
Provided is an aqueous composition for ophthalmic or nasal administration comprising levocetirizine or its salt. In one embodiment, the composition comprises levocetirizine or its salt at a levocetirizine concentration of 0.05-0.5% (w / v). The composition may further comprise a surface active agent and / or an organic acid salt.
Owner:NITTO MEDIC CO LTD
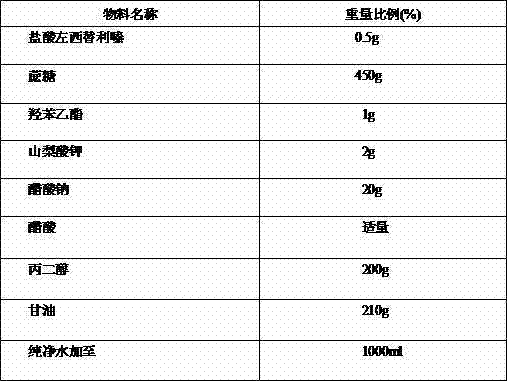
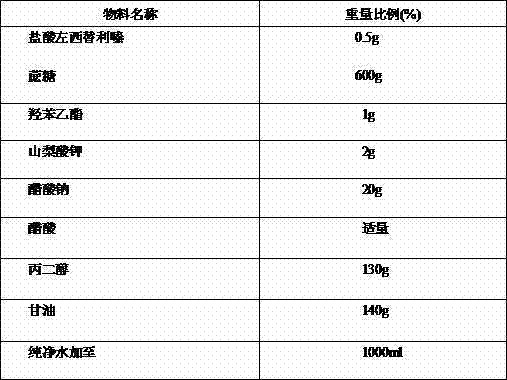
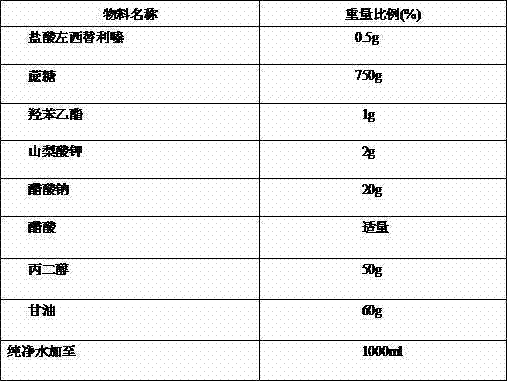
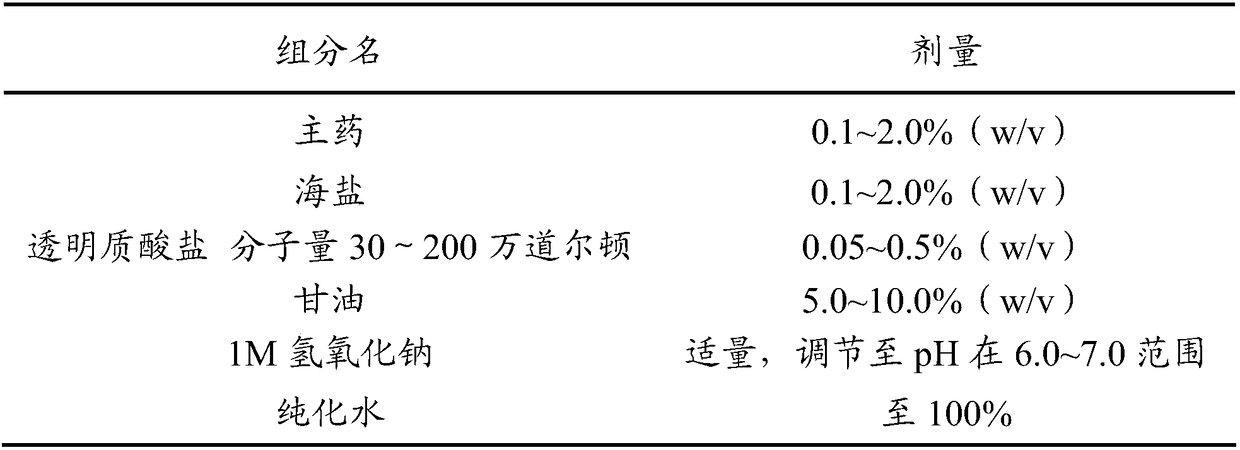
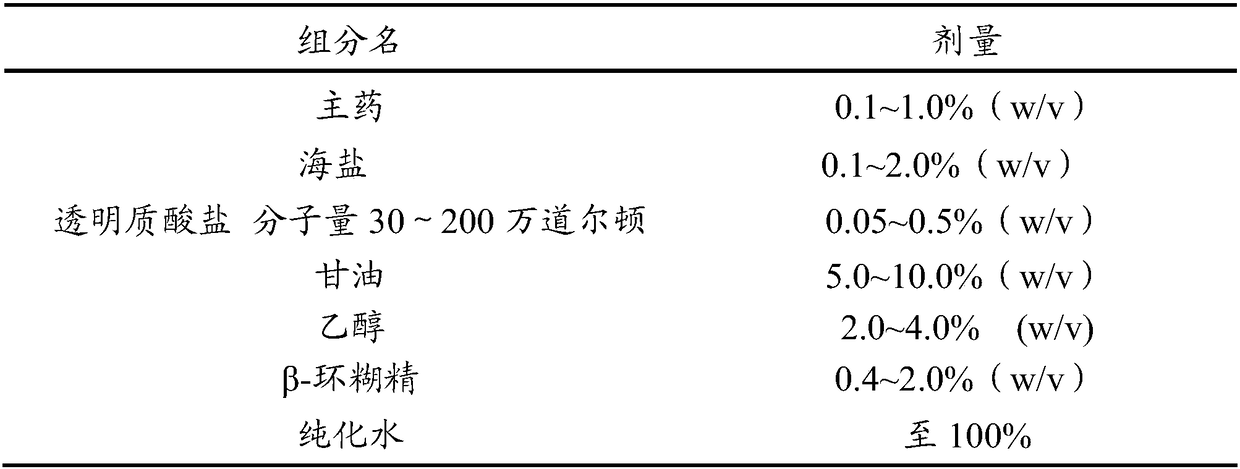
Antiallergic nasal medicine composition with high moisture retention and preparation methods and applications thereof
InactiveCN109276715AUse lessImprove comfortPharmaceutical delivery mechanismPharmaceutical non-active ingredientsIrritationGlycerol
The invention provides an antiallergic nasal medicine composition with high moisture retention and preparation methods and applications thereof. The composition has high moisture retention and no irritation to a nasal mucosa. The composition comprises levocetirizine, azatadine, loratadine, ebastine, setastine, bilastine, clemastine, mizolastine, epinastine and moxifloxacin which are antiallergic medicine as main medicine, wherein the main medicine can be salts or free alkalis, sea salt as an osmotic pressure regulator, and a mixture of sodium hyaluronate and glycerin of different molecular weights as a moisturizing excipient. Plant essential oil can be contained in the composition. Preparations can be an aqueous solution, a cyclodextrin-coated suspension or a nanosuspension. Dosage forms include spray, aerosol and nose drops. Main indications include nasal dryness, runny nose, nasal itching, nasal obstruction and the like due to allergic rhinitis.
Owner:XIAN LIBANG PHARMA TECH
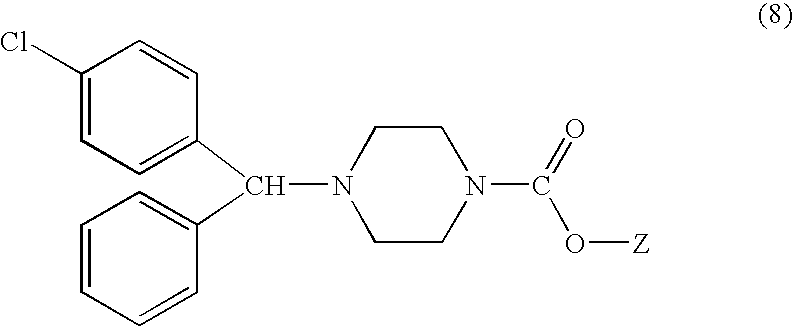
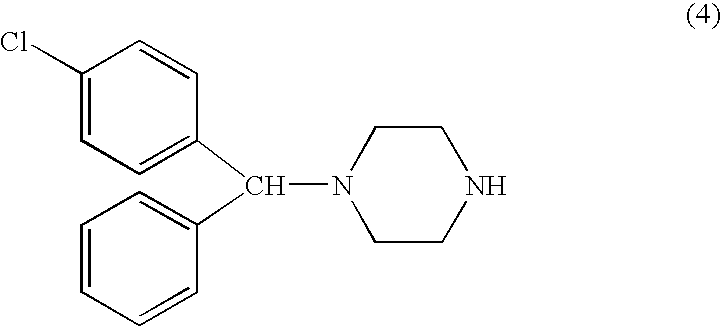
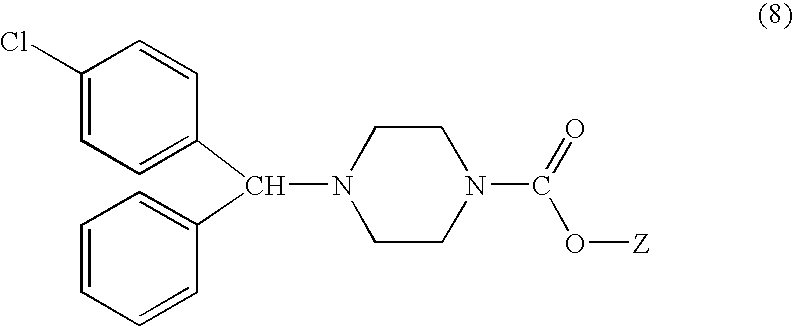
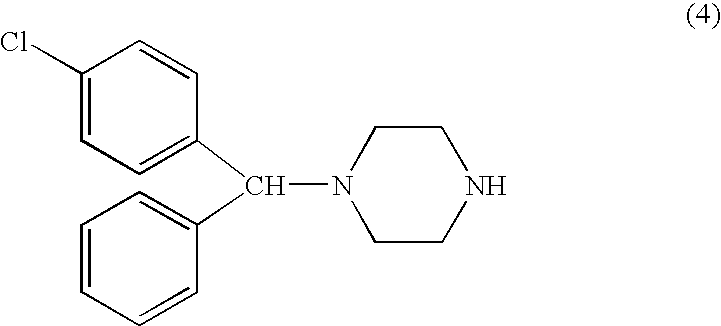
Process for making n-(diphenylmethyl)piperazines
The compound of formula (8), in racemic or single enantiomeric form, is useful in making N-(diphenylmethyl)-piperazines such as cetirizine and levocetrizine.wherein Z is preferably phenyl.
Owner:SYNTHON BV
Process for making n-(diphenylmethyl)piperazines
The compound of formula (8), in racemic or single enantiomeric form, is useful in making N-(diphenylmethyl)-piperazines such as cetirizine and levocetrizine.wherein Z is preferably phenyl.
Owner:SYNTHON BV
Complex granule formulation having improved stability comprising levocetirizine and montelukast
InactiveUS20160367551A1Improve bioavailabilityImproved patient complianceSenses disorderGranular deliveryLevocetirizineAlkalizing agent
The present invention relates to a complex granule formulation comprising a first granular part comprising levocetirizine or its pharmaceutically acceptable salt, cyclodextrin or its derivative, and an alkalinizing agent; and a second granular part comprising montelukast or its pharmaceutically acceptable salt, cyclodextrin or its derivative, and an alkalinizing agent. This formulation can effectively inhibit the production of related compounds of levocetirizine and montelukast by allowing levocetirizine and montelukast to form clathrate complexes with cyclodextrin, and using an alkalinizing agent. This formulation not only shows increased stability and bioavailability, but also improves patient compliance owing to its effective masking of bitter taste.
Owner:HANMI PHARMA
Method for separating and determining levocetirizine hydrochloride and genotoxic impurity E thereof by HPLC method
ActiveCN110988163AEasy to separateIncreased durabilityComponent separationHplc methodMonopotassium phosphate
The invention belongs to the field of analytical chemistry, and particularly relates to a method for separating and determining levocetirizine hydrochloride and a genotoxic impurity E thereof by an HPLC method. A chromatographic column adopted in the method takes octadecyl bonded silica gel as a filler, a mixed mobile phase of potassium dihydrogen phosphate and methanol and / or acetonitrile is adopted for elution, and a product enters a detector for detection. The method is a reversed-phase high performance liquid chromatography method. Separation and detection of levocetirizine hydrochloride and genotoxic impurities E thereof can be realized at the same time; the method has excellent separation performance and durability, is simple, convenient and feasible, has good reproducibility, is efficient and rapid, can achieve very good effects on tailing factors and theoretical pedal numbers, can effectively determine the content of genotoxic impurities E in levocetirizine hydrochloride, and has strong specificity.
Owner:CHONGQING HUABANGSHENGKAI PHARM
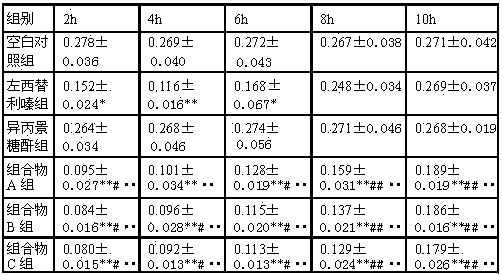
Pharmaceutical composition for treating acetyl cholinergic urticaria
ActiveCN103690555AWeak anti-allergic effectImprove anti-allergicOrganic active ingredientsDermatological disorderPharmaceutical drugTherapeutic effect
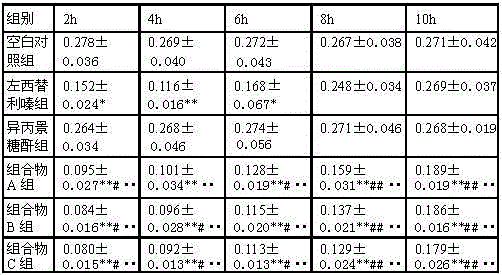
The invention belongs to the technical field of treatment medicines for urticaria, and specially relates to a pharmaceutical composition for treating acetyl cholinergic urticaria and application of the pharmaceutical composition in preparation of a medicine for preventing or treating the acetyl cholinergic urticaria, aiming at overcoming the shortage that the existing medicine for treating the acetyl cholinergic urticaria has poor treatment effect and cannot act for a long time in the prior art. The pharmaceutical composition for treating or preventing the acetyl cholinergic urticaria consists of levocetirizine and isopropylidene sedoheptuiosan. The pharmaceutical composition shows a good effect on treatment of acetyl cholinergic urticaria and is remarkably worthy of clinical popularization.
Owner:启东市清汉农副产品专业合作社
Pharmaceutical composition for treating acute urticaria
InactiveCN103585160AGood synergyImprove anti-allergicOrganic active ingredientsImmunological disordersAcute urticariaSide effect
The invention discloses pharmaceutical composition for treating acute urticaria and an application of the pharmaceutical composition to preparation of drugs for preventing or treating the acute urticaria, and aims at overcoming the defect that drugs for treating the acute urticaria are poor in effect and non-sustainable in action at present, the pharmaceutical composition for preventing or treating the acute urticaria is low in treatment cost, convenient to take by a patient, quick in curative effect and free of side effects and comprises levocetirizine and triptolide. The pharmaceutical composition has a good treatment effect in treating the acute urticaria and obvious clinical promotional values.
Owner:李伟丽
A kind of levocetirizine injection
ActiveCN107961215BAlleviate and eliminate allergic reactionsNo undesired hemolysisOrganic active ingredientsAutomatic syringesCetirizine HydrochlorideBiochemistry
The invention discloses a new dosage form of levocetirizine injection and a method for preparing levocetirizine injection. A levocetirizine, pH regulator, osmotic pressure regulator and other raw materials are used to prepare a levocetirizine injection. A cetirizine hydrochloride injection with stable content, low impurity content, and long validity period. The levocetirizine injection prepared by the present invention is safe and effective, can effectively relieve and eliminate allergic reactions, and has no unwanted hemolytic reactions, filling This kind of technology gap at home and abroad has been filled.
Owner:COSCI MED TECH CO LTD
A kind of hplc method for separating and measuring levocetirizine hydrochloride and its genotoxic impurity e
The invention belongs to the field of analytical chemistry, and in particular relates to a method for separating and measuring levocetirizine hydrochloride and its genotoxic impurity E by HPLC. The chromatographic column adopted in the method is filled with octadecyl bonded silica gel, and is eluted with a mixed mobile phase of potassium dihydrogen phosphate, methanol and / or acetonitrile, and enters a detector for detection. The method is reversed-phase high-performance liquid chromatography, which can realize the separation and detection of levocetirizine hydrochloride and its genotoxic impurity E at the same time, and has excellent separation performance and durability, is simple and feasible, has good reproducibility, and is highly efficient. Fast, tailing factor and theoretical number of steps can achieve very good results, and can effectively determine the content of genotoxic impurity E in levocetirizine hydrochloride, with strong specificity.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Aqueous composition for ophthalmic or nasal administration
Provided is an aqueous composition for ophthalmic or nasal administration comprising levocetirizine or its salt. In one embodiment, the composition comprises levocetirizine or its salt at a levocetirizine concentration of 0.05-0.5% (w / v). The composition may further comprise a surface active agent and / or an organic acid salt.
Owner:NITTO MEDIC CO LTD
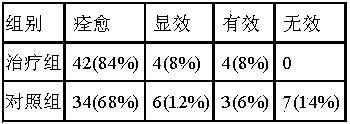
Compound medicine composition for treating urticaria
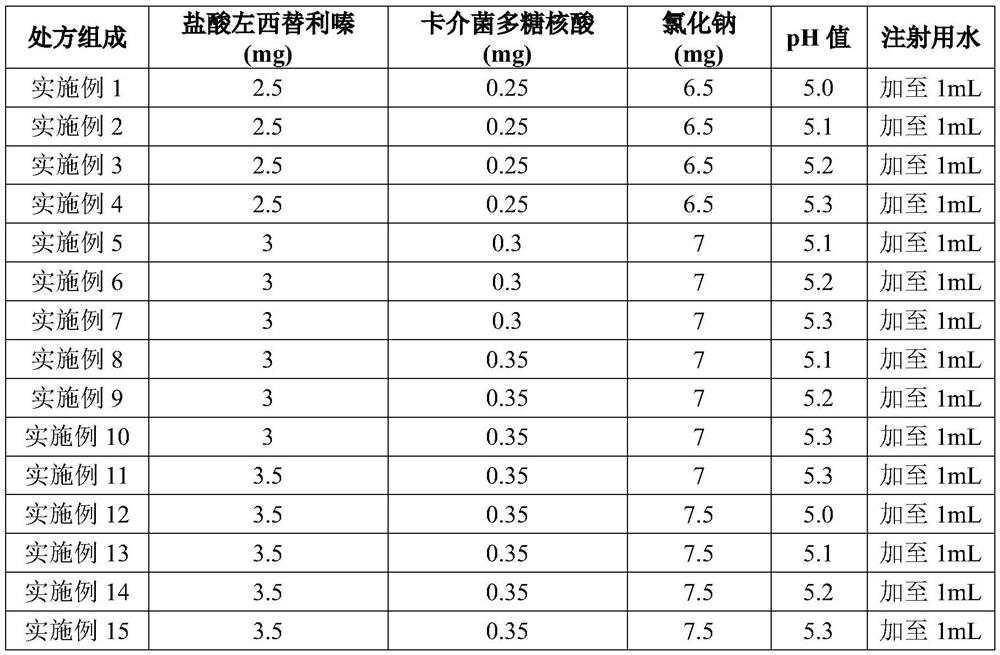
PendingCN114404443AImprove stabilityImprove complianceOrganic active ingredientsInorganic non-active ingredientsPharmaceutical drugCurative effect
The compound pharmaceutical composition for treating urticaria comprises two active pharmaceutical ingredients, namely levocetirizine hydrochloride and bacillus calmette-guerin polysaccharide nucleic acid, and the weight ratio of levocetirizine hydrochloride to bacillus calmette-guerin polysaccharide nucleic acid is (5-25): 1. Tests prove that the pharmaceutical composition disclosed by the invention has a good curative effect on urticaria caused by various reasons, the efficacy of the composition is obviously superior to that of levocetirizine hydrochloride or an immunomodulator when the levocetirizine hydrochloride or the immunomodulator is independently applied, and the pharmaceutical composition shows very excellent storage stability.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
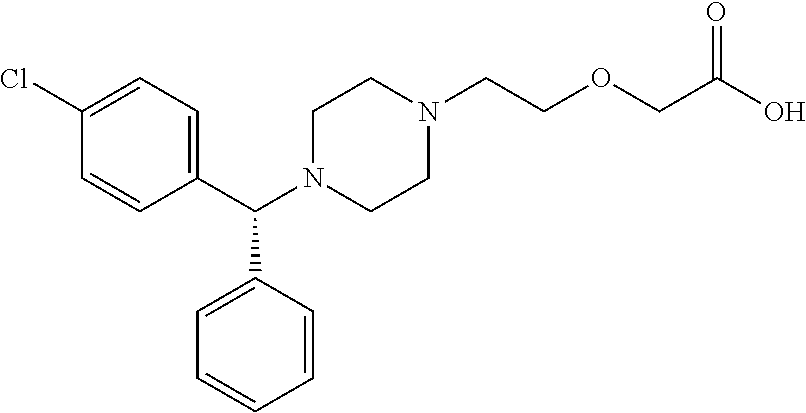
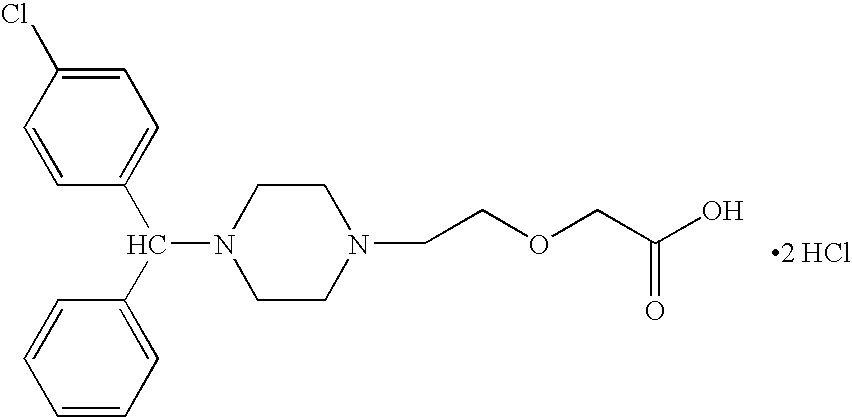
Process For The Preparation Of Levocetirizine And Intermediates Thereof
The present invention describes a novel process for the preparation of levocetirizine and pharmaceutically acceptable acid addition salts thereof using diglycolic acid or derivatives thereof and new intermediates used in that process.
Owner:KRKA TOVARNA ZDRAVIL D D
Levocetirizine hydrochloride eye drop composition and preparation method thereof
PendingCN113712907AImprove antibacterial efficacyReduce dosageOrganic active ingredientsInorganic non-active ingredientsOPHTHALMOLOGICALSAntiseptic Agent
The invention relates to the technical field of ophthalmic medicines, in particular to levocetirizine hydrochloride eye drops and a preparation method thereof. The levocetirizine hydrochloride eye drops comprise the following components in parts by weight: 15-18 parts of levocetirizine hydrochloride, 50-55 parts of an osmotic pressure regulator, 50-55 parts of a thickening agent, 8-11 parts of a pH regulator and 0.01-0.02 part of a preservative; the pH value of the components is 7.0 to 7.8, and the osmotic pressure molar concentration of the components is 280 mOsmol / kg to 320 mOsmol / kg. According to the invention, the problem of compatibility of benzalkonium chloride and a plastic bottle in the existing levocetirizine hydrochloride eye drops is effectively solved, related substances are obviously superior to those of the existing levocetirizine hydrochloride eye drops, and the long-term stability is better; the dosage of the preservative is less, which is more environment-friendly and low in consumption; the number of contact lens patients is increased, and the medication compliance is improved.
Owner:南京帝昌医药科技有限公司
Use of levocetirizine and montelukast in the treatment of traumatic injury
InactiveCN105517631AExtended treatment periodShorten healing timeAntibacterial agentsNervous disorderMontelukastInjury brain
The embodiments described herein include methods and formulations for treating lung and brain injury. The methods and formulations include, but are not limited to, methods and formulations for delivering effective concentrations of levocetirizine and montelukast to a patient in need. The methods and formulations can comprise conventional and / or modified-release elements, providing for drug delivery to the patient.
Owner:INFLAMMATORY RESPONSE RES
Complex granule formulation having improved stability comprising levocetirizine and montelukast
InactiveUS9801876B2Improve stabilityImprove bioavailabilitySenses disorderGranular deliveryMontelukastPatient compliance
The present invention relates to a complex granule formulation comprising a first granular part comprising levocetirizine or its pharmaceutically acceptable salt, cyclodextrin or its derivative, and an alkalinizing agent; and a second granular part comprising montelukast or its pharmaceutically acceptable salt, cyclodextrin or its derivative, and an alkalinizing agent. This formulation can effectively inhibit the production of related compounds of levocetirizine and montelukast by allowing levocetirizine and montelukast to form clathrate complexes with cyclodextrin, and using an alkalinizing agent. This formulation not only shows increased stability and bioavailability, but also improves patient compliance owing to its effective masking of bitter taste.
Owner:HANMI PHARMA
A kind of method for separating and measuring levocetirizine hydrochloride and related substances by high performance liquid chromatography
ActiveCN110726788BEasy to separateStrong specificityComponent separationFluid phasePolyethylene glycol
The invention relates to a method for separating and measuring levocetirizine hydrochloride and five related substances by high-performance liquid chromatography. The chromatographic column of the method is a C18 chromatographic column. The mobile phase was eluted and detected, and alkali was also added to the mobile phase. The method can simultaneously quantify levocetirizine hydrochloride, levocetirizine lactose, levocetirizine polyethylene glycol ester, p-chlorobenzophenone, p-chlorodicarbonate in levocetirizine hydrochloride tablets at the same time. Any one or more of the five related substances of benzyl alcohol and p-chlorobenzylpiperazine are separated and detected. Compared with the prior art, the separation degree of levocetirizine hydrochloride and various related substances is better, the sensitivity is high, and the separation and detection efficiency is improved.
Owner:HUNAN JIUDIAN PHARMA
Dosing regimen for injectable cetirizine
ActiveUS11253513B2Organic active ingredientsPharmaceutical delivery mechanismDosing regimenCetirizine
Described herein is a method of administering injectable cetirizine or levocetirizine including intravenously injecting a mammal in need thereof with a therapeutically effective amount of an injectable cetirizine or levocetirizine composition, wherein the mammal receives a maximum daily dose of the cetirizine or levocetirizine that is no more than 15 times a maximum recommended daily clinical dose for the mammal.
Owner:JDP THERAPEUTICS LLC
Pharmaceutical composition for treating acetyl cholinergic urticaria
ActiveCN103690555BWeak anti-allergic effectImprove anti-allergicOrganic active ingredientsDermatological disorderPharmaceutical drugTherapeutic effect
The invention belongs to the technical field of treatment medicines for urticaria, and specially relates to a pharmaceutical composition for treating acetyl cholinergic urticaria and application of the pharmaceutical composition in preparation of a medicine for preventing or treating the acetyl cholinergic urticaria, aiming at overcoming the shortage that the existing medicine for treating the acetyl cholinergic urticaria has poor treatment effect and cannot act for a long time in the prior art. The pharmaceutical composition for treating or preventing the acetyl cholinergic urticaria consists of levocetirizine and isopropylidene sedoheptuiosan. The pharmaceutical composition shows a good effect on treatment of acetyl cholinergic urticaria and is remarkably worthy of clinical popularization.
Owner:启东市清汉农副产品专业合作社
Levocetirizine hydrochloride injection and preparation method thereof
PendingCN114306227ASubstance reductionImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismCyclodextrin DerivativesPulmonary edema
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com