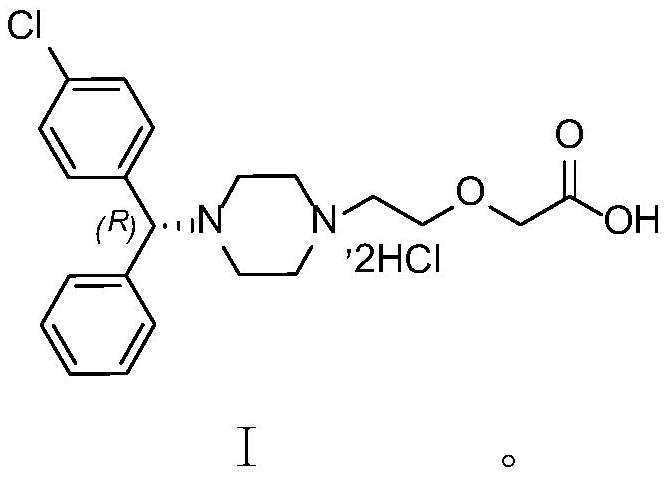
A kind of hplc method for separating and measuring levocetirizine hydrochloride and its genotoxic impurity e
A technology for levocetirizine hydrochloride and genotoxicity, applied in the field of analytical chemistry, to achieve the effects of strong specificity, good separation, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Mobile phase A: Weigh potassium dihydrogen phosphate 2.72 (0.02mol / L potassium dihydrogen phosphate) in a 1000ml volumetric flask, add water to dissolve and dilute to the mark, and adjust the pH value to 3.0±0.1 with phosphoric acid;
[0082] Mobile phase B: acetonitrile;
[0083] The volume ratio of mobile phase A and mobile phase B is 95:5.
[0084] (1) Preparation of solution
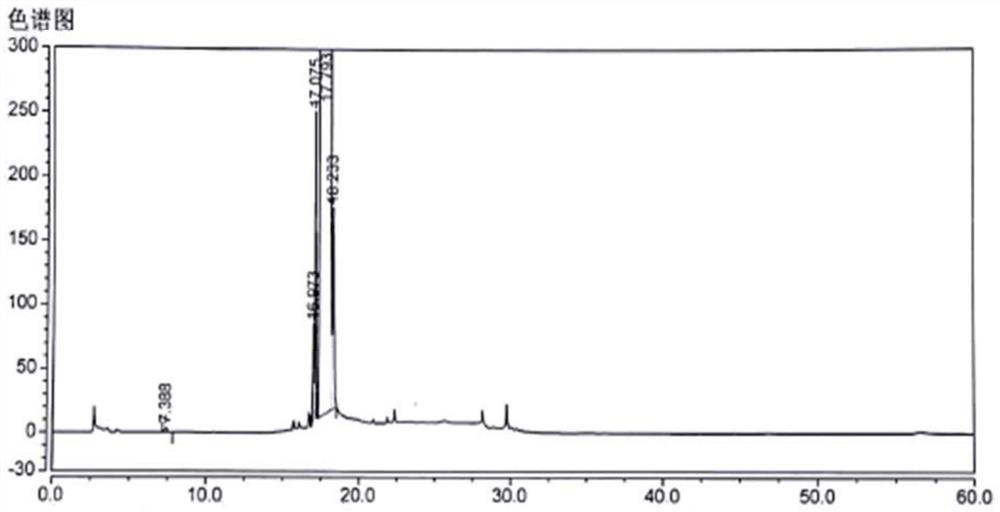
[0085] Preparation of impurity E solution: take about 25 mg of impurity E reference substance, put it in a 25ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and obtain impurity E stock solution.
[0086] Impurity E reference substance solution preparation: Precisely pipette 0.6ml of the above solution, put it in a 100ml measuring bottle, add diluent to dilute to the mark, and shake well to obtain a reference substance solution with an impurity E concentration of 6 μg / ml.
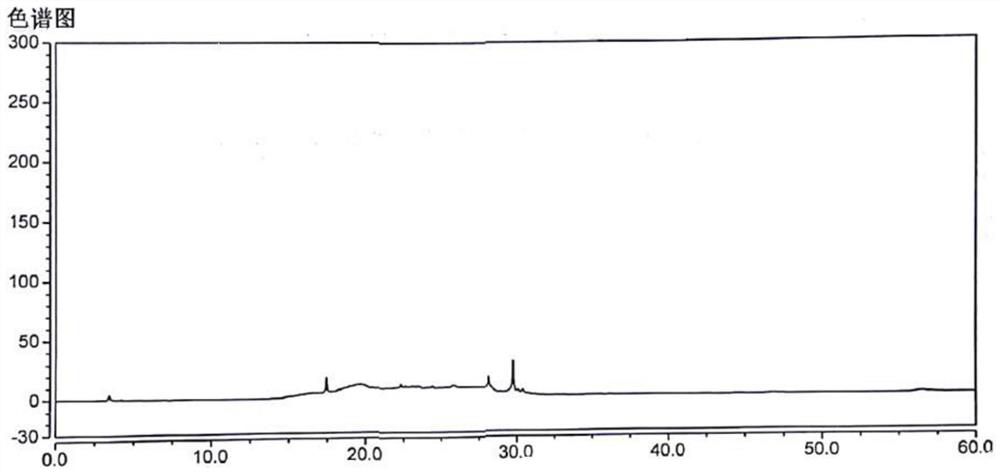
[0087] The preparation of levocetirizine hydrochloride need testing solution: take by w...
Embodiment 2 comparative example 1
[0094] Mobile phase A: water.
[0095] Mobile phase B: acetonitrile;
[0096] The volume ratio of mobile phase A and mobile phase B is 95:5.
[0097] Get the need testing solution prepared in embodiment 1 step 1) by above-mentioned chromatographic condition sampling, record chromatogram, measurement result is shown in Table 2.
[0098] Table 2 test results
[0099]
[0100] Conclusion: Different from mobile phase A in Example 1, the peak separation between impurity E and levocetirizine hydrochloride is 9.00, but the peak shape of impurity E is not good, not sharp, and the peak eluting time is late, so the sensitivity cannot meet the requirements. Adding salts to the mobile phase can advance the peak time and have a better peak shape.
Embodiment 3 comparative example 2
[0102] Mobile phase A: Weigh 0.92g of formic acid (0.02mol / L formic acid) into a 1000ml volumetric flask, add water to dissolve and dilute to the mark, and adjust the pH value to 3.0±0.1 with phosphoric acid;
[0103] Mobile phase B: acetonitrile;
[0104] The volume ratio of mobile phase A and mobile phase B is 95:5.
[0105] Get the need testing solution prepared in embodiment 1 step (1) by above-mentioned chromatographic condition sampling, record chromatogram, measurement result is shown in Table 3.
[0106] Table 3 test results
[0107]
[0108] Conclusion: In the separation test with the same other conditions as in the above example, except that the mobile phase A is different, the peak of impurity E appears, and the peak of levocetirizine hydrochloride does not appear, but the peak shape of impurity E is not good, not sharp, and the sensitivity cannot be satisfied Require. It is better to add salts to the mobile phase as buffer salts than to add acids as buffer sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com