Method for separating and measuring levocetirizine dihydrochloride and related substances by using high performance liquid chromatography
A technology of levocetirizine hydrochloride and high performance liquid chromatography, applied in the field of medical analysis, can solve the problems of inability to accurately quantify, interfere with the detection of levocetirizine lactose ester, etc., and achieve improved efficiency, strong specificity, and good separation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 The inventive method detects related substances in levocetirizine hydrochloride tablets
[0073] The chromatographic column is a C18 chromatographic column (150mm×4.6mm, 5μm); the mobile phase is sodium heptanesulfonate solution: acetonitrile=62:38, the preparation method of sodium heptanesulfonate solution is to take 1.0g sodium heptanesulfonate, add water Dissolve and dilute to 1000ml, add 0.3ml triethylamine, adjust pH value to 3.5 with 0.5mol / L sulfuric acid solution.
[0074] Preparation of the test solution: take 10 tablets of this product, put them in a 200ml measuring bottle, add about 160ml of mobile phase, shake to dissolve levocetirizine hydrochloride, dilute to the mark with mobile phase, shake well, filter, and take the subsequent filtrate As the test solution;
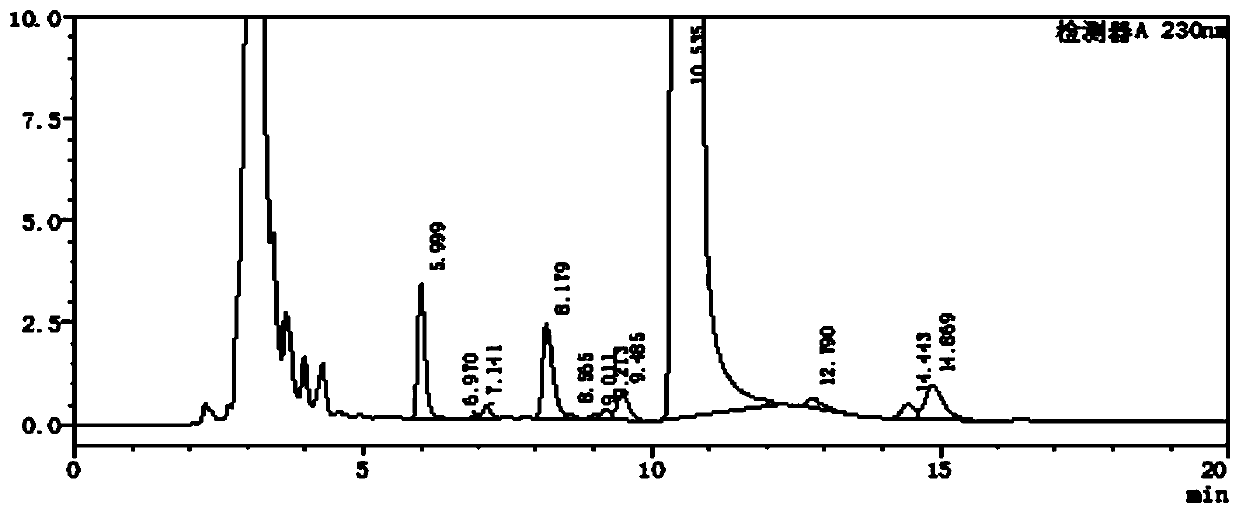
[0075] Reference substance solution preparation: Accurately weigh cetirizine hydrochloride lactose ester reference substance, p-chlorobenzhydryl piperazine reference substance, cetiri...
Embodiment 2
[0082] Example 2 This method detects related substances in levocetirizine hydrochloride tablets
[0083] The chromatographic column is a C18 chromatographic column (150mm×4.6mm, 5μm); the mobile phase is sodium heptanesulfonate solution: acetonitrile=62:38, the preparation method of sodium heptanesulfonate solution is to take 1.0g sodium heptanesulfonate, add water Dissolve and dilute to 1000ml, add 0.3ml diethylamine, adjust pH value to 3.5 with 0.5mol / L sulfuric acid solution.
[0084] Need testing solution and reference substance solution are consistent with embodiment 1.
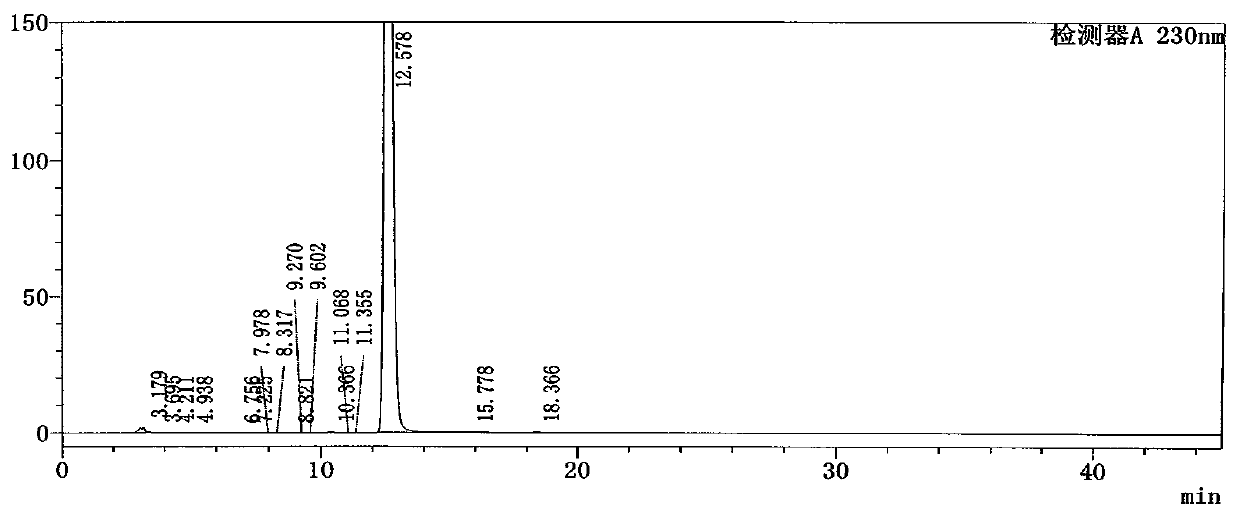
[0085] Get need testing solution, each 20ul of reference substance solution inject liquid chromatograph respectively, carry out elution by chromatographic condition of the present invention, the flow velocity of mobile phase is 1.5ml / min, and detection wavelength is 230mg, and chromatographic column column temperature is 30 ℃, Record the chromatogram, attached Image 6 . Details are shown in Table 10 ...
Embodiment 3
[0091] Example 3 This method detects related substances in levocetirizine hydrochloride tablets
[0092] The chromatographic column is a C18 chromatographic column (150mm×4.6mm, 5μm); the mobile phase is sodium heptanesulfonate solution: acetonitrile=62:38, the preparation method of sodium heptanesulfonate solution is to take 1.0g sodium heptanesulfonate, add water Dissolve and dilute to 1000ml, add 0.3ml ammonia water, adjust pH value to 3.5 with 0.5mol / L sulfuric acid solution.
[0093] Need testing solution and reference substance solution are consistent with embodiment 1.
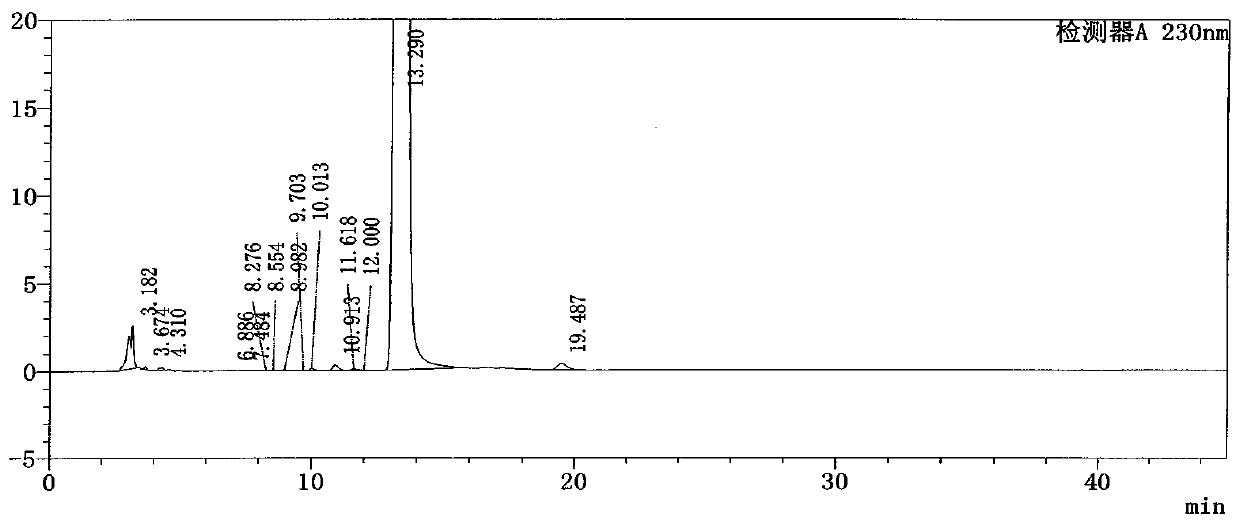
[0094] Get need testing solution, each 20ul of reference substance solution inject liquid chromatograph respectively, carry out elution by chromatographic condition of the present invention, the flow velocity of mobile phase is 1.5ml / min, and detection wavelength is 230mg, and chromatographic column column temperature is 30 ℃, Record the chromatogram, attached Figure 7 . Details are shown in Table 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com