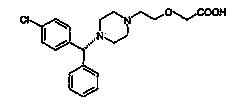
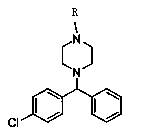
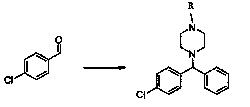
Novel synthetic process for levocetirizine and key intermediates
A technology of levocetirizine and compounds, applied in the direction of organic chemistry, can solve the problems of unsatisfactory purity and yield of levocetirizine, and achieve the effects of convenient use, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1, the preparation of the compound of compound formula (II)
[0063] Add 50 g of 1,4-oxocyclohexane, 80 g of thionyl chloride, and 20 g of potassium hydrogen persulfide into the reaction vessel, and react at room temperature for 1 hour, then slowly add 10 g of anhydrous acetic acid, and then add 3 g of sodium hypochlorite, and react 3 hours, filtered, the filter cake was dissolved by adding 100 g of water, and extracted twice with 80 g of acetone respectively; the organic phases were combined, filtered and concentrated under reduced pressure to obtain the title compound.
Embodiment 2
[0064] Embodiment 2, the preparation of the compound of chemical formula (Ⅲ)
[0065] In the reaction vessel, 10 g of the product obtained in Example 1 was added with 100 g of anhydrous piperazine, then triethylamine was added, and 40 g of dichloromethane and 50 g of p-toluenesulfonyl chloride were added dropwise at room temperature, and the reaction 2 hours, the organic phase was separated, washed with 20 g of saturated brine, dried, filtered, and concentrated under reduced pressure to obtain the title compound.
Embodiment 3
[0066] Embodiment 3, the preparation of the compound of chemical formula (Ⅳ)
[0067] Put 20g of the product obtained in Example 2 into a reaction vessel, add 15g of bromobenzene, 60ml of acetonitrile, and 12g of cobalt bromide, then stir the mixture and heat it up to 70-80°C, keep it warm for 1.5 hours, and add p-toluenesulfonate dropwise Acyl piperazine 20g, keep warm for 5 hours after dropping, concentrate under reduced pressure, recover acetonitrile, filter and dry to obtain the title compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com