Preparation method of C10-C13 long-chain normal paraffin hydrocarbon dehydrogenation catalyst supporter
A C10-C13, catalyst carrier technology, applied in the direction of catalyst carrier, metal/metal oxide/metal hydroxide catalyst, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
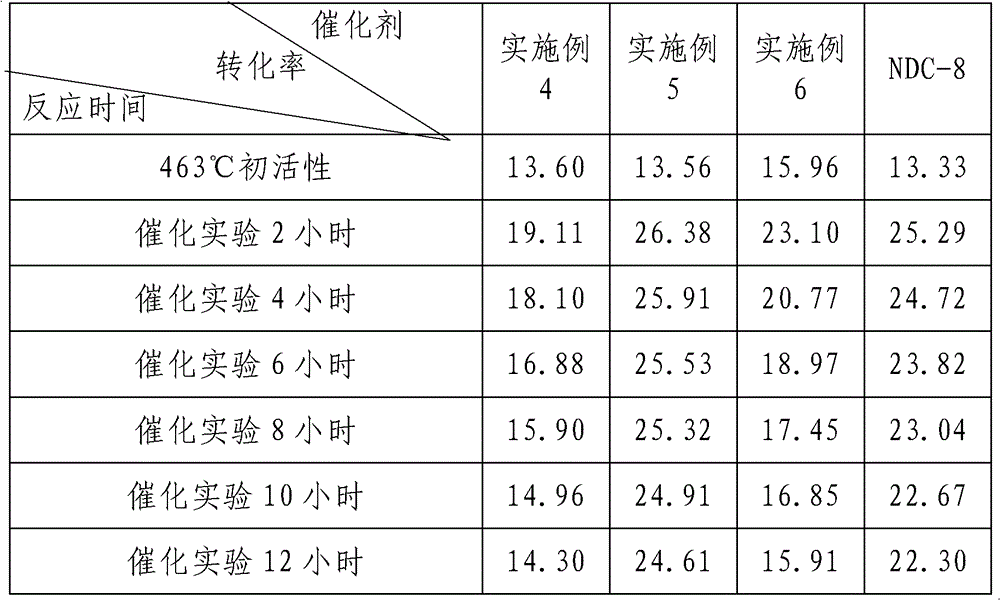
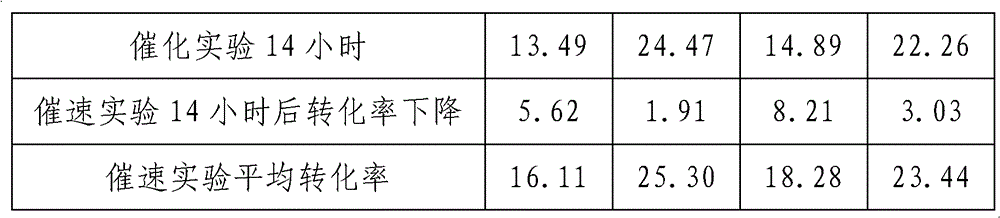
Image
Examples
Embodiment 1
[0021] Example 1: MgAl 2 O 4 Preparation of the vector
[0022] Weigh Al(NO 3 ) 3 ·9H 2 O 187.5g1 and Mg(NO 3 ) 2 ·6H 2 O 64.1g is dissolved in 1L water, the temperature of the solution is controlled by a water bath at 40°C, then the concentrated ammonia solution is added dropwise to the solution and vigorously stirred (stirring speed 350r / min), until the pH of the solution reaches 10, the dropwise addition of ammonia water is stopped, and the stirring is stable for 60 minutes , the stirring solution was stopped at 40 °C for 1 h and then filtered. Washed with an equal volume of deionized water, the filtered colloid was extruded into strips, dried at a constant temperature, and then placed in an oven at 105 °C for 12 hours. The dried solid was heated in a muffle furnace at a heating rate of 1-20 / min. After reaching 600°C, the corresponding carrier was prepared by constant temperature calcination for 4 hours.
Embodiment 2
[0023] Example 2: Preparation of support for magnesium-aluminum composite
[0024] Weigh Al(NO 3 ) 3 ·9H 2 O 220.0g and Mg(NO 3 ) 2 ·6H 2 O 40.0g is dissolved in 1L water, the temperature of the solution is controlled by a water bath at 40°C, then the concentrated ammonia solution is added dropwise to the solution and vigorously stirred (stirring speed 350r / min), until the pH of the solution reaches 10, stop dripping ammonia water, and stir to stabilize for 60 minutes , the stirring solution was stopped at 40 °C for 1 h and then filtered. Washed with an equal volume of deionized water, the filtered colloid was extruded into strips, dried at a constant temperature, and then placed in an oven at 105 °C for 12 hours. The dried solid was heated in a muffle furnace at a heating rate of 1-20 / min. After reaching 600°C, the corresponding carrier was prepared by constant temperature calcination for 4 hours.
Embodiment 3
[0025] Example 3: Preparation of Alumina Support
[0026] Weigh Al(NO 3 ) 3 ·9H 2 O 250.0g was dissolved in 1L water, the temperature of the solution was controlled by a water bath at 40°C, then the concentrated ammonia solution was added dropwise to the solution and vigorously stirred (stirring speed 350r / min), until the pH of the solution reached 10, the dropwise addition of ammonia was stopped, and the stirring was stable for 60 minutes , the stirring solution was stopped at 40 °C for 1 h and then filtered. Washed with an equal volume of deionized water, the filtered colloid was extruded into strips, dried at a constant temperature, and then placed in an oven at 105 °C for 12 hours. The dried solid was heated in a muffle furnace at a heating rate of 1-20 / min. After reaching 600°C, the corresponding carrier was prepared by constant temperature calcination for 4 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com