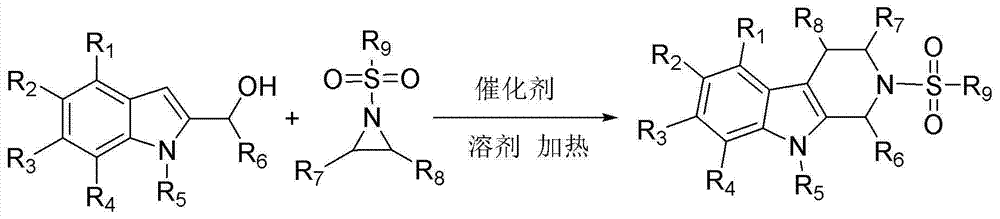
Method for preparing N-sulfonyl substituted tetrahydro-beta-carboline derivative
A technology for sulfonyl and derivatives, which is applied in the field of N-sulfonyl-substituted tetrahydro-β-carboline derivatives and their preparation, and achieves the effects of high yield and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
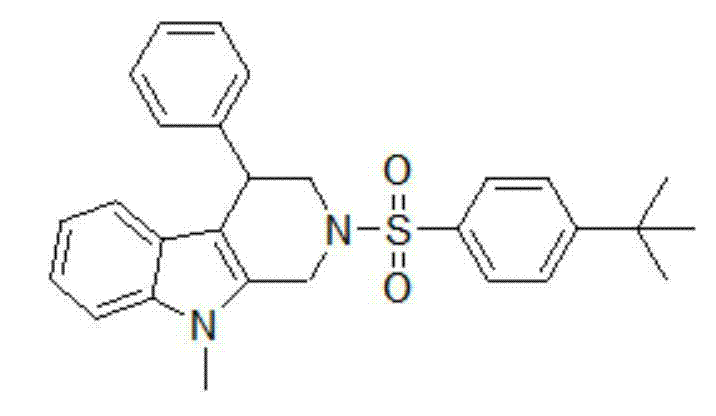
Embodiment 1
[0018] Add N-methylindole-2-methanol (0.6 mmol), 2-phenyl-1-p-toluenesulfonyl aziridine (0.5 mmol), scandium trifluorosulfonate (0.01 mmol) and ClCH2CH2Cl (3 mL), then heated to 83 °C and stirred for 2 hours (TLC monitoring). After the reaction, distilled water (10 mL) was added to terminate the reaction, extracted with ethyl acetate (30 mL × 3), dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was mixed with petroleum ether: ethyl acetate = 4:1 The solvent was the developing solvent, and the product (154 mg, yield: 74%) was isolated by column chromatography.
[0019] Its structural formula is:
[0020]
Embodiment 2
[0022] Add N-methylindole-2-methanol (0.6 mmol), 2-p-fluorophenyl-1-p-toluenesulfonyl aziridine (0.5 mmol), scandium trifluorosulfonate (0.01 mmol) to the reaction flask in sequence ), ClCH2CH2Cl (3 mL), then heated to 83 °C and stirred for 2 hours (monitored by TLC). After the reaction, distilled water (10 mL) was added to terminate the reaction, extracted with ethyl acetate (30 mL × 3), dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was mixed with petroleum ether: ethyl acetate = 4:1 The solvent was a developing solvent, and the product (167 mg, yield: 77%) was isolated by column chromatography.
[0023] Its structural formula is:
[0024]
Embodiment 3
[0026] Add N-methylindole-2-methanol (0.6 mmol), 2-p-chlorophenyl-1-p-toluenesulfonyl aziridine (0.5 mmol), scandium trifluorosulfonate (0.01 mmol) to the reaction flask in turn ), ClCH2CH2Cl (3 mL), then heated to 83 °C and stirred for 2 hours (monitored by TLC). After the reaction, distilled water (10 mL) was added to terminate the reaction, extracted with ethyl acetate (30 mL × 3), dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was mixed with petroleum ether: ethyl acetate = 4:1 The solvent was a developing solvent, and the product (169 mg, yield: 75%) was isolated by column chromatography.
[0027] Its structural formula is:
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com