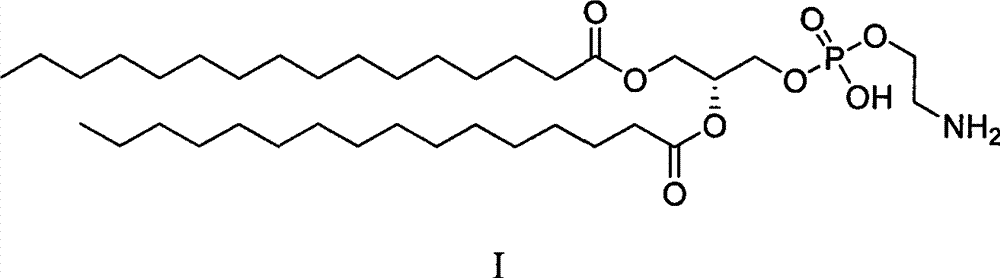
Synthetic method of 2-dipalmitoyl-sn-glycero-3-phosphoethanolamine
A technology of dipalmitoylphosphatidylethanolamine and its synthesis method, which is applied in the direction of phosphorus organic compounds, etc., and can solve the problems of many impurities, unsuitable for large-scale industrial production, and long reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
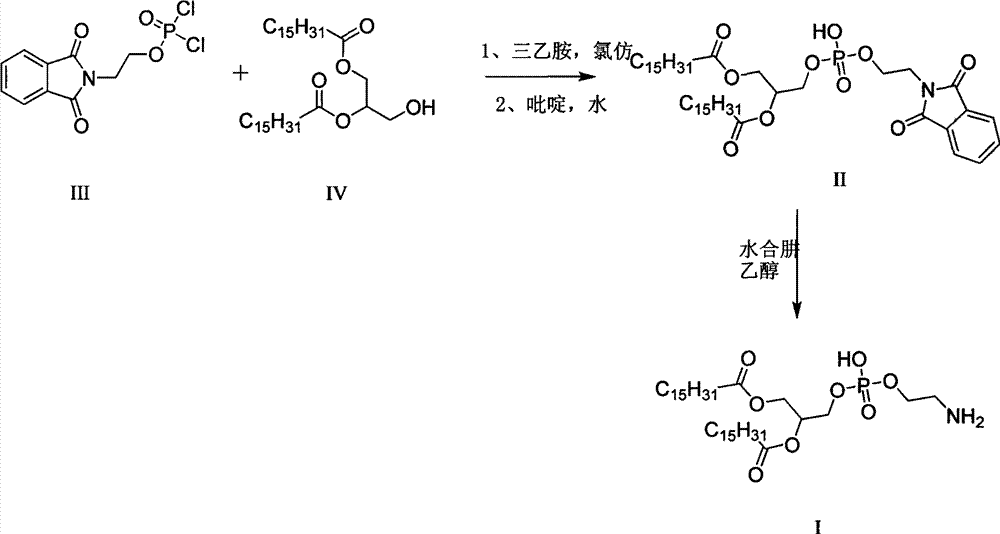
[0021] Synthesis of Intermediate II
[0022] Add compound III (28.2 g, 0.09 mol) into the reactor, add anhydrous tetrahydrofuran (80 ml), stir and dissolve, and set aside. Take another reactor, add compound IV (40g, 0.07mol) and molecular sieve-dried pyridine (200ml), heat to 40°C to dissolve it completely, and cool to room temperature. The solution of compound IV was added dropwise to the standby solution in a cold bath at 8°C, and the reaction was continued at 10°C for 35 minutes after the drop was completed, and the reaction was completed. Add 6N hydrochloric acid dropwise at 10°C to adjust the pH to 1-2, stir for 3 minutes after the dropwise addition, and stand for 3 hours for crystallization. Filter, add water to the filter cake and stir wash it once, filter, and oven dry at 40°C to obtain 49.8g of intermediate II, with a purity of 93.9% and a yield of 86.2%.
[0023] Synthesis of dipalmitoylphosphatidylethanolamine
[0024] Intermediate II (49.8g) was added into the r...
Embodiment 2
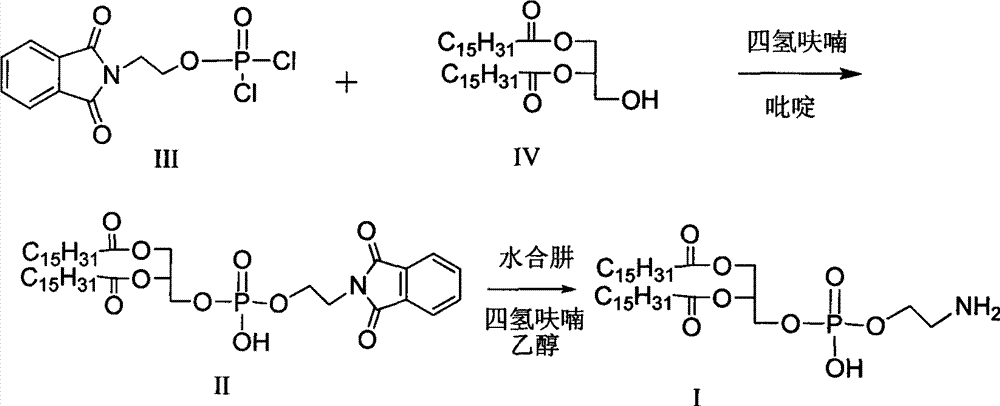
[0026] Synthesis of Intermediate II
[0027] Add compound III (140 g, 0.454 mol) into the reactor, add anhydrous tetrahydrofuran (400 ml), stir to dissolve, and set aside. Take another reactor, add compound IV (200g, 0.35mol) and molecular sieve-dried pyridine (800ml), heat to 40°C to make it fully dissolved, and cool to room temperature. The solution of compound IV was added dropwise to the standby solution in a cold bath at 10°C, and the reaction was continued at 10°C for 70 minutes after dropping, and the reaction was completed. Add 6N hydrochloric acid dropwise at 10°C to adjust the pH to 1-2, stir for 3 minutes after the dropwise addition, and stand for 2 hours for crystallization. Filtrate, add water to the filter cake and stir wash once, filter, and oven-dry at 40°C to obtain 252.9 g of Intermediate II with a purity of 95.1% and a yield of 87.5%.
[0028] Synthesis of dipalmitoylphosphatidylethanolamine
[0029] Add intermediate II to the reactor, add tetrahydrofuran...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com