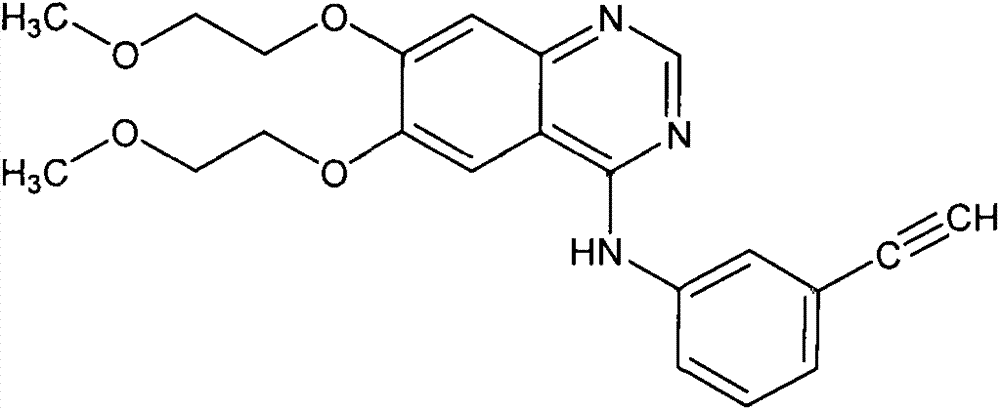
Erlotinib hydrochloride tablets and preparation method thereof
An erlotinib hydrochloride tablet and a technology for erlotinib hydrochloride, which are applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of dust flying, increase drug production costs, Problems such as adverse effects on the health of production workers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
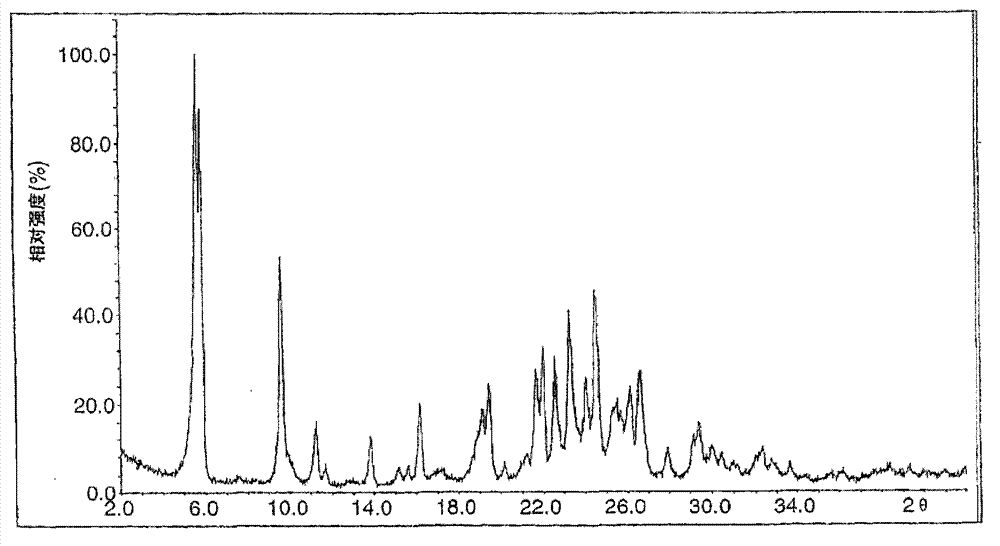
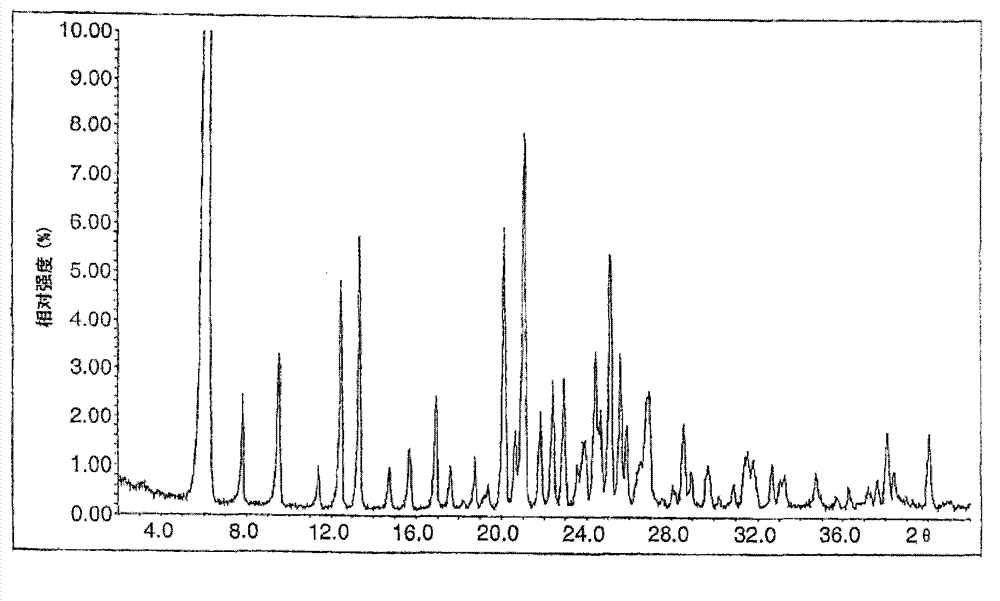
Image
Examples
Embodiment 1-4 and comparative Embodiment 5
[0026] Table 1
[0027]
[0028] Preparation Process:
[0029] 1. will RH40 (or Tween 20, Tween 40, SDS) mixed with lactose;
[0030] 2. Mix the materials except magnesium stearate with the mixture obtained in step "1";
[0031] 3. Carry out dry granulation of the material obtained in step "2";
[0032] 4. Add magnesium stearate to mix, and compress into tablets;
[0033] 5. The tablet is coated with a coating powder with the trade name of Opadry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com