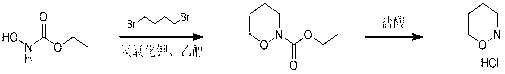
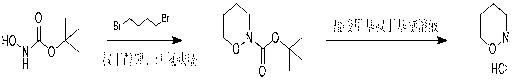
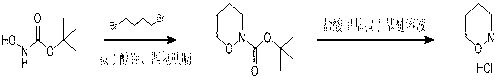Method of synthesizing 1, 2-morpholine hydrochloride
A technology of morpholine hydrochloride and hydroxylamine hydrochloride is applied in the field of synthesis of 1,2-morpholine hydrochloride, can solve the problems of high synthesis cost, complicated operation, expensive synthesis raw materials, etc. Easy to scale up, cost-effective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Dissolve 10 g (75 mmol, 1.0 eq) of N-tert-butoxycarbonyl-hydroxylamine in 200 mL of THF, then add 150 mmol (2.0 eq) of potassium tert-butoxide, and stir at room temperature for 1 hour . Then, a THF solution (50 mL) of 1,4-dibromobutane (17.8 g, 82.5 mmol, 1.1 eq) was added dropwise to the reaction solution, heated to reflux and stirred for 12 hours. Then the reaction solution was cooled, and saturated ammonium chloride solution was added, extracted with ethyl acetate (200 ml*3), the organic phase was washed twice with saturated brine, the organic phase was combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure 13.5 g of crude N-tert-butoxycarbonyl-1,2-cyclobutanolamine were obtained.
[0013] Dissolve 13.5 g of crude N-tert-butoxycarbonyl-1,2-cyclobutanolamine in 100 mL methyl tert-butyl ether, and add 20 mL methyl tert-butyl ether hydrogen chloride gas-saturated solution dropwise. Stir at room temp...
Embodiment 2
[0014] Example 2: Dissolve 10 g (75 mmol) N-tert-butoxycarbonyl-hydroxylamine in 200 mL of THF, then add 188 mmol (2.5 eq ) potassium tert-butoxide, and stir at room temperature for 1 hour. Then, a THF solution (50 mL) of 1,4-dibromobutane (17.8 g, 82.5 mmol, 1.1 eq) was added dropwise to the reaction solution, and heated to reflux with stirring for 12 hours. Then the reaction solution was cooled, a saturated solution of ammonium chloride was added, extracted with ethyl acetate (200 ml*3), the organic phase was washed with saturated brine, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 14.5 g of crude N-tert-butoxycarbonyl-1,2-cyclobutanolamine.
[0015] Dissolve 14.5 g of crude N-tert-butoxycarbonyl-1,2-cyclobutanolamine in 100 mL methyl tert-butyl ether, and add 20 mL methyl tert-butyl ether hydrogen chloride gas-saturated solution dropwise. Stir at room temperature for 3 ho...
Embodiment 3
[0016] Example 3: Example 2: Dissolve 10 g (75 mmol) N-tert-butoxycarbonyl-hydroxylamine in 200 mL of THF, then add 225 mmol (3.0 eq ) potassium tert-butoxide, and stir at room temperature for 1 hour. Then, a THF solution (50 mL) of 1,4-dibromobutane (17.8 g, 82.5 mmol, 1.1 eq) was added dropwise to the reaction liquid, and heated to reflux with stirring for 14 hours. Then the reaction solution was cooled, a saturated solution of ammonium chloride was added, extracted with ethyl acetate (200 ml*3), the organic phase was washed with saturated brine, the combined organic phases were filtered over anhydrous sodium sulfate, and the filtrate was concentrated under reduced pressure to obtain 14.8 g of Crude N-tert-butoxycarbonyl-1,2-cyclobutanolamine.
[0017] Dissolve 14.8 g of crude N-tert-butoxycarbonyl-1,2-cyclobutanolamine in 100 mL of methyl tert-butyl ether, and add 20 mL of methyl tert-butyl ether hydrogen chloride gas-saturated solution dropwise. Stir at room temperature f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com