Time resolution fluorescence method comprehensive detection pancreatic cancer kit and application thereof
A time-resolved fluorescence, comprehensive detection technology, applied in the field of biological and medical detection technology, can solve the problems of far different judgment results, errors in detection results, and many detection steps, and achieves fewer steps, shorter time, and improved specificity. and sensitivity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
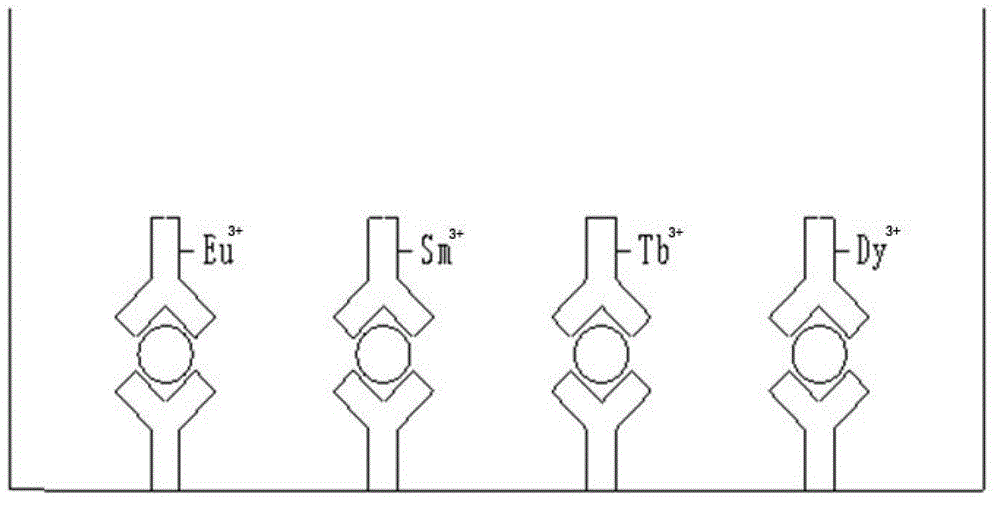
[0033] The time-resolved fluorescence method comprehensive detection kit for pancreatic cancer provided in this example includes four monoclonal antibodies: anti-CA242 antibody C3918, anti-CA50 antibody AM09238PU-N, anti-CA199 antibody ab3982, and anti-CEA antibody MAM02-008 Composition of mixed antibody-coated microplates; with Eu 3+ Labeled C3624 antibody (anti-CA242 antibody), with Tb 3+ Labeled AM09239PU-N antibody (anti-CA50 antibody), with Sm 3+ Labeled ab116024 antibody (anti-CA199 antibody), with Dy 3+ Labeled MAM02-881 antibody (anti-CEA antibody) mixed antibody composed of four monoclonal antibodies; analysis buffer; common fluorescence enhancer; washing solution; quality control composed of four standard mixtures of CA242, CA50, CEA, and CA199 Taste. The kit also includes negative controls, positive controls, substrates and other related reagents.
[0034] In the kit provided in this example, anti-CA242 antibodies C3918 and C3624 were self-made; anti-CA199 antib...
Embodiment 2
[0047] The application of the kit provided in Example 1 of the present invention in the comprehensive detection of pancreatic cancer tumor markers CA242, CA50, CEA, and CA199, that is, the kit provided in Example 1 of the present invention is used to comprehensively detect pancreatic cancer tumor markers CA242, CA242, and CA199. CA50, CEA, CA199 time-resolved fluorescent immunoassay method, the reaction principle is as follows figure 1 As shown, the double-antibody sandwich method is used, including the following steps:
[0048] (1) Dilute the four protein standard mixtures of CA242, CA50, CEA, and CA199 with the analysis buffer, that is, the quality control product, and make a mixed solution with a series of concentration gradients containing the four protein standards of CA242, CA50, CEA, and CA199 ;
[0049](2) Take 100 μl human serum of the sample to be tested and the mixed solution of four protein standards prepared in step (1), add the mixed antibody-coated antibody com...
Embodiment 3
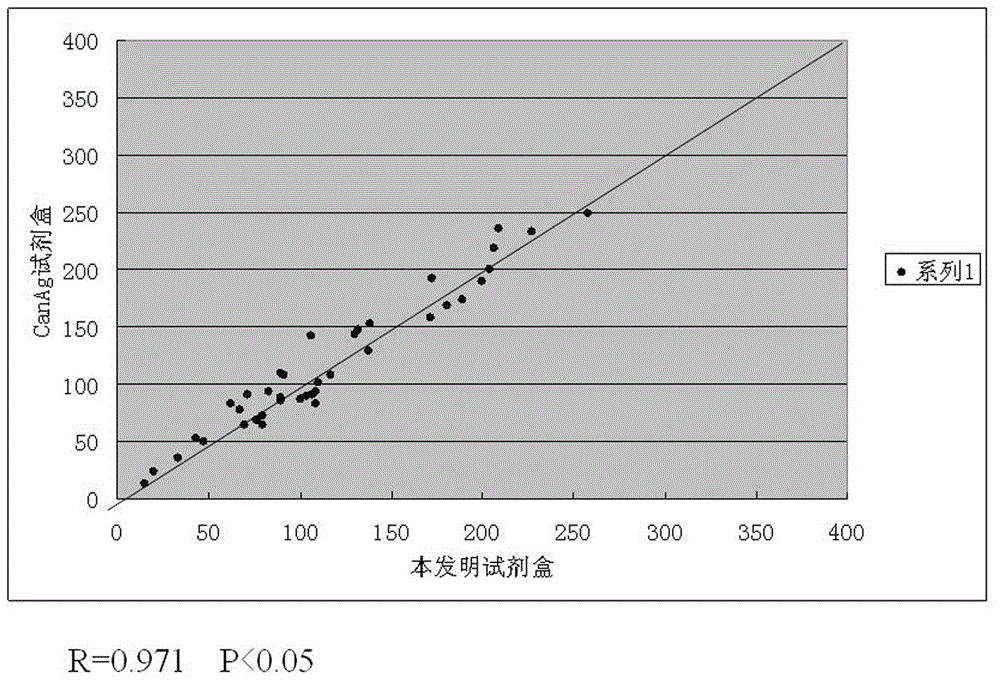
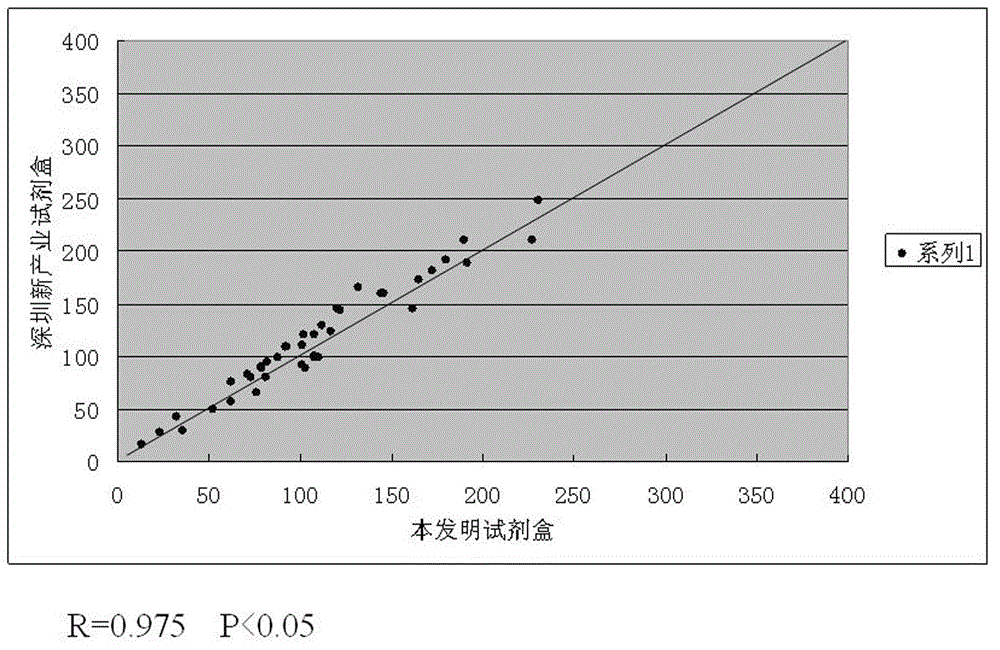
[0051] The numerical values of CA242, CA50, CEA, and CA199 four indexes obtained by testing 40 serum samples with the test kit of the present invention and the same 40 serum samples are respectively tested with the single-index kit of CanAg Company for CA242 and Shenzhen New Industry Company. Regression correlation analysis was performed on the data obtained from the single-index kit test CA50, the single-index kit test CA199 of Shenzhen New Industry Company, and the single-index kit test CEA of Abbott Company, respectively, corresponding to the attached Figure 2-5 , as can be seen from the accompanying drawings, each index data obtained by the test kit of the present invention has a good correlation with the data obtained by the test of the single index kit respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com