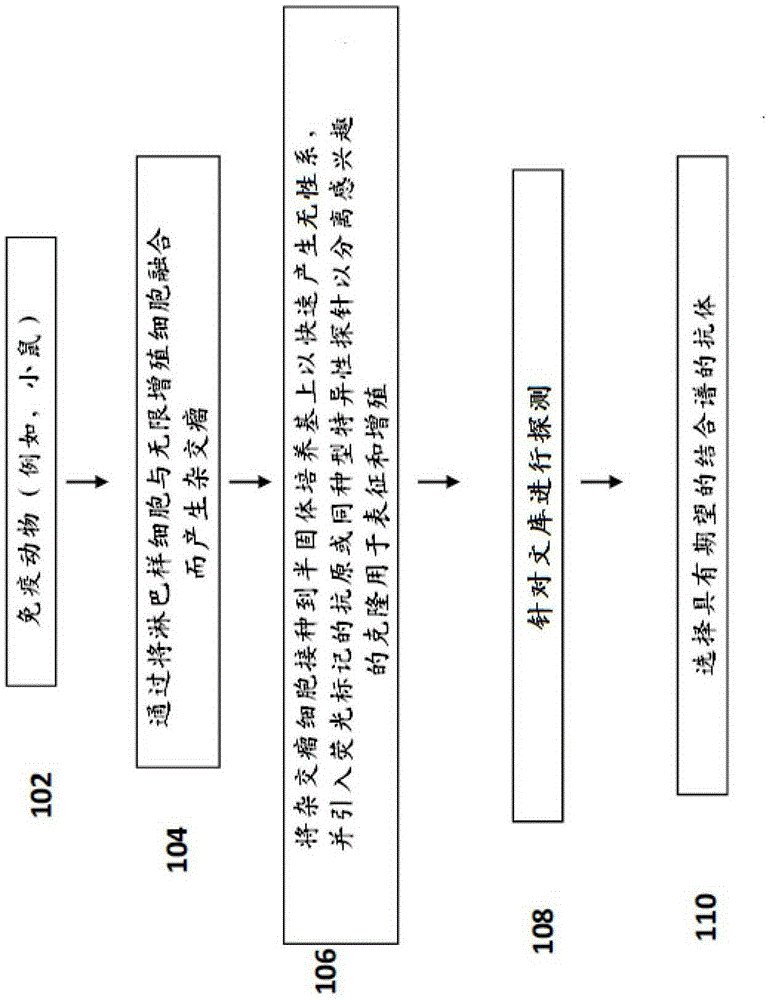
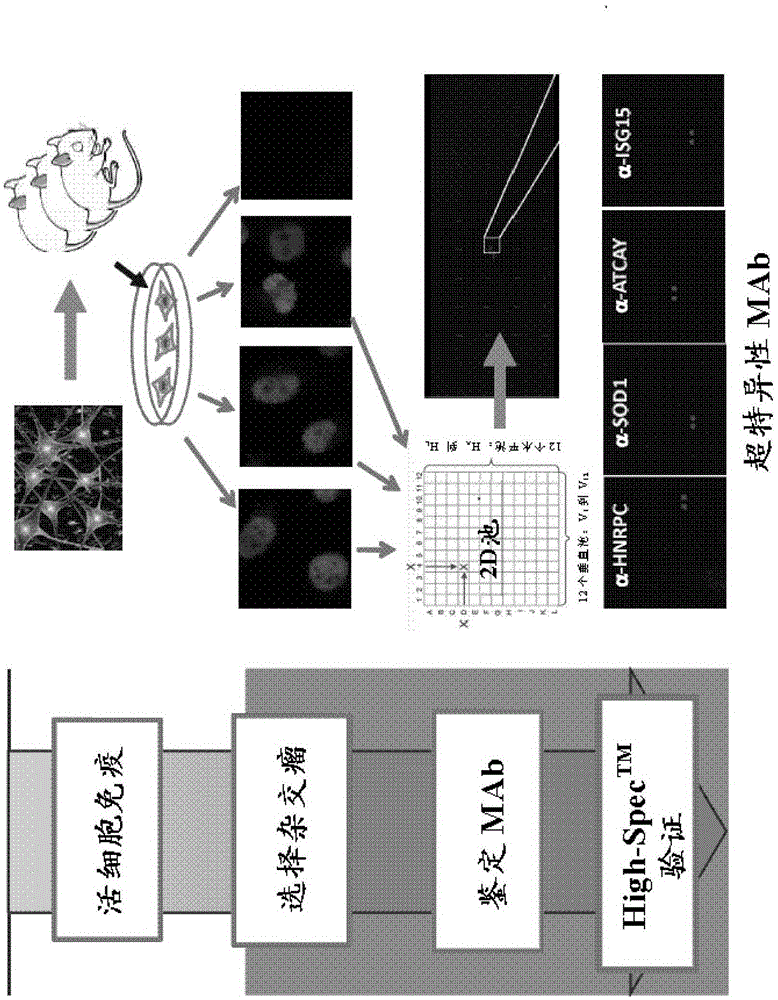
Methods and systems for generating, validating and using monoclonal antibodies
A monoclonal antibody, antibody technology, applied in the field of characterization and utilization of antibodies, production, production, can solve the problems of expensive and time-consuming antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0200] Example 1: Subcloning of approximately 17,000 full-length human ORFs into expression vectors .
[0201] Obtained approximately 16,000 unique ORF's ORF collection (Invitrogen, CA), while an additional 1,000 full-length human ORFs were subcloned. constructed for yeast Compatible expression vector (pEGH-A), which produces N-terminal 6xHis6::glutathione-S-transferase (GST) fusion protein in yeast after galactose induction ( image 3 ). Subsequently, all approximately 17,000 human ORFs were subcloned with a success rate of 99% as determined by restriction digestion. In particular, three replicates were prepared for this pool, from one of which the entry DNA (EntryDNA) was extracted, and the quality of the plasmid DNA was checked on an agarose gel. The resulting recombinants were then transformed into bacteria and single colonies were selected on plates containing Amp. For each LR recombinant, four single colonies were picked to generate glycerol stocks, two of which...
Embodiment 2
[0203] Example 2: Design and construction of a microarray of 5000 human antigens.
[0204] Preliminary experiments were performed to test the ability to rapidly purify properly folded recombinant proteins for microarray production. These proteins fall into 5 distinct functional classes: transcription factors and transcription co-regulators, RNA-binding proteins, protein kinases, chromatin-associated and chromatin-modifying proteins, and mitochondrial proteins. Proteins were placed into these categories based on primary sequence, literature and Gene Ontology annotations. Expressed ORFs represent up to 85% (in terms of transcription factors) of all human proteins in the relevant functional class. More than 90% of expressed proteins were purified at levels sufficient for array construction (Ho, S.W., et al., Linking DNA-binding proteins to their recognition sequences by using protein microarrays. Proc Natl Acad Sci USA, 2006. 103(26): p.9940-5).
[0205] Several functional as...
Embodiment 3
[0206] Embodiment 3: the preparation of human antigen protein chip
[0207] To purify the full set of 17,000 human protein antigens from yeast cells, the entire human ORF master set was cloned into yeast in pEGH-A, single colonies were picked and glycerol stocks were prepared. Yeast cells were induced for recombinant protein production and stored at -80°C. To monitor the quality of the induced cultures, 24 random strains were inoculated in duplicate for each batch of culture preparations and first underwent a protein purification step. Using immunoblotting and silver staining techniques, the success rate of culture induction was estimated ( Figure 4 A). Success was only indicated when at least 85% of the purified protein showed a major band within the expected molecular weight range in immunoblot and silver stain analysis. Otherwise, repeat the culture preparation for the failed batch. Using this standard, all 17,000 antigenic proteins were purified with an 85% success ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com