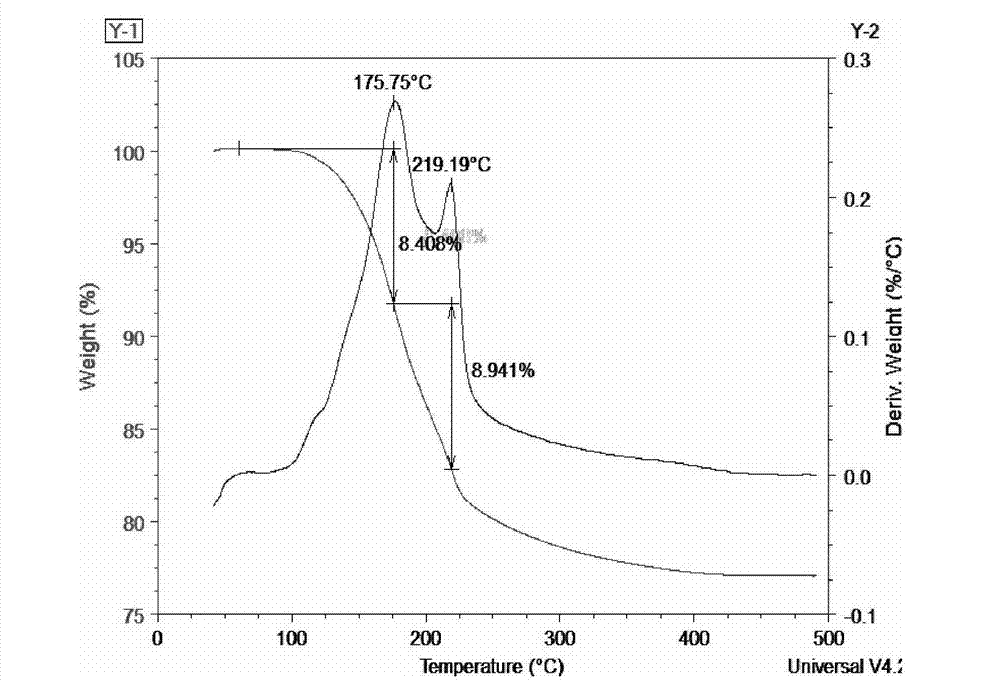
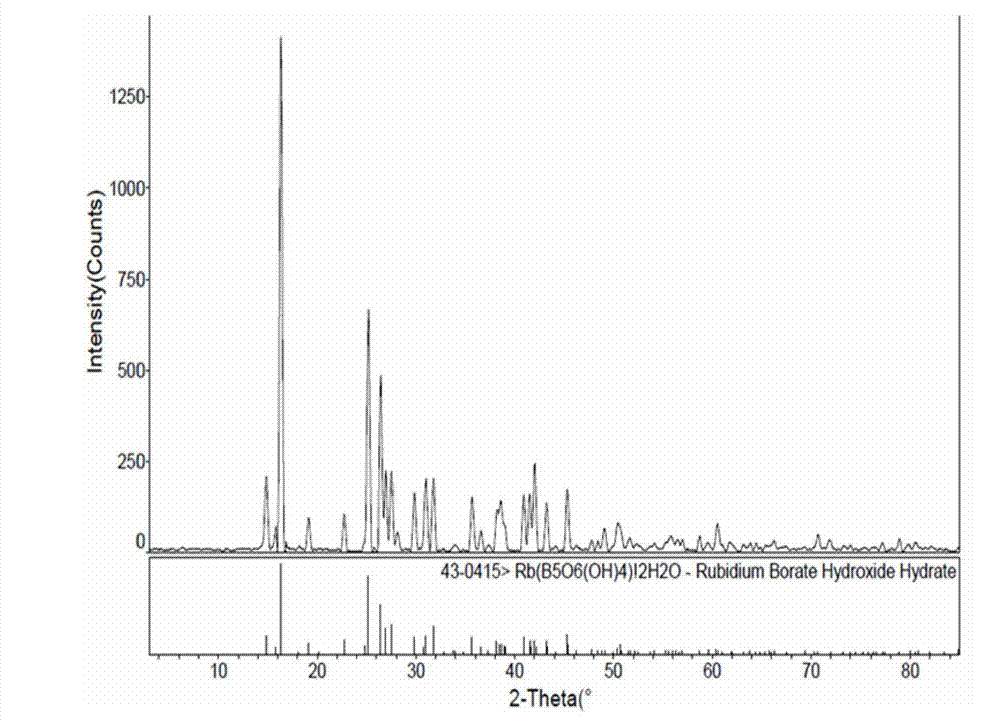
Method for preparing rubidium pentaborate
A technology of pentaboric acid and rubidium carbonate, applied in the directions of borates, boron oxy compounds, etc., can solve the problems of many by-products, low yield, long synthesis time, etc., and achieve the advantages of short synthesis time, high yield and shortened reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of rubidium pentaborate of the present invention comprises the steps:
[0028] a, rubidium carbonate is added in the boric acid solution of 20~30wt%, stirs, obtains reaction liquid; Wherein, the weight of rubidium carbonate is 7.5~11.5% of the water weight in the boric acid solution;
[0029] b. Filter the reaction solution obtained in step a, and dry the filter cake to obtain final product.
[0030] The reaction formula of boric acid and rubidium carbonate solution in the inventive method is as follows:
[0031] Rb 2 CO 3 +H 3 BO 3 +H 2 O→Rb[B 5 o 6 (OH) 4 ]·2H 2 O+CO 2 ↑+H 2 o
[0032] Among them, rubidium carbonate is very easy to absorb water. In order to increase the yield, as a preferred solution, it needs to be dried before using rubidium carbonate to remove water. As a more preferred solution, rubidium carbonate should be heated at 150-200 ° C before adding the boric acid solution. Dry to constant weight.
[0033] Further, on...
Embodiment 1
[0041] Embodiment 1 adopts the inventive method to prepare rubidium pentaborate
[0042] a. Add rubidium carbonate to a 20wt% boric acid solution at 70°C and stir to obtain a reaction solution; wherein, rubidium carbonate is dried at 150°C to constant weight before adding the boric acid solution, and the weight of rubidium carbonate is equal to that in the boric acid solution 7.5% of the water weight; the preparation method of the boric acid solution is: dry the boric acid at 70°C to constant weight, then add it into the water with a temperature of 70°C according to the proportion, dissolve it, and prepare it; the reaction temperature of rubidium carbonate and boric acid 70°C;
[0043] b. Filter the reaction solution obtained in step a, and dry the filter cake at 70°C to obtain the product.
[0044] It has been determined that the synthesis time of rubidium pentaborate in this embodiment is 19 hours in total, and the yield of rubidium pentaborate is 98.06%.
Embodiment 2
[0045] Embodiment 2 adopts the inventive method to prepare rubidium pentaborate
[0046] a. Add rubidium carbonate to a 20wt% boric acid solution at 75°C and stir to obtain a reaction solution; wherein, rubidium carbonate is dried at 180°C to constant weight before adding the boric acid solution, and the weight of rubidium carbonate is equal to that in the boric acid solution 7.5% of water weight; the preparation method of boric acid solution is: dry boric acid at 75°C to constant weight, then add it into water with a temperature of 75°C according to the proportion, dissolve it, and prepare it; the reaction temperature of rubidium carbonate and boric acid 75°C;
[0047] b. Filter the reaction solution obtained in step a, and dry the filter cake at 75°C to obtain the product.
[0048] It has been determined that the synthesis time of rubidium pentaborate in this embodiment is 18 hours in total, and the yield of rubidium pentaborate is 98.85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com