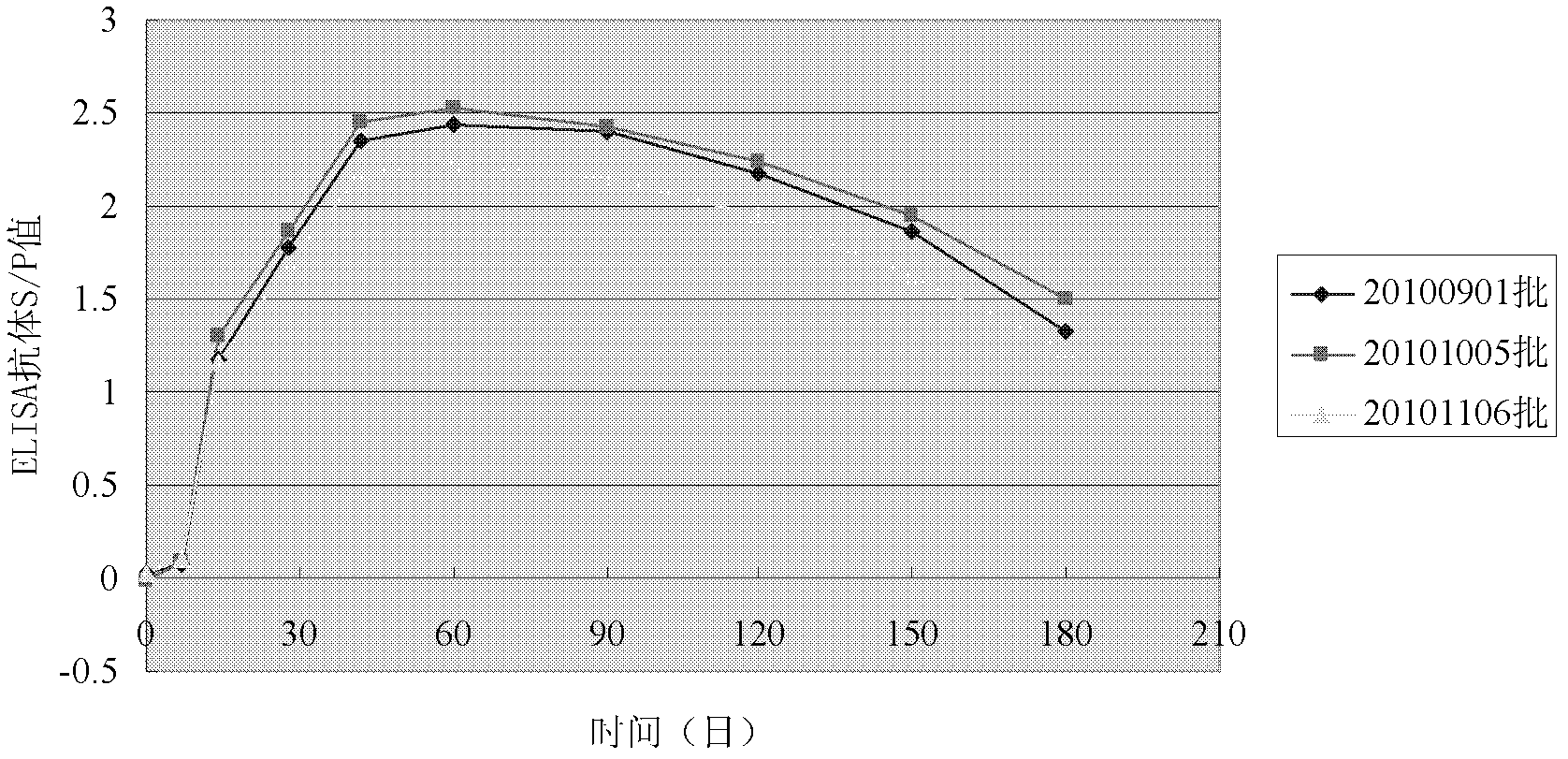
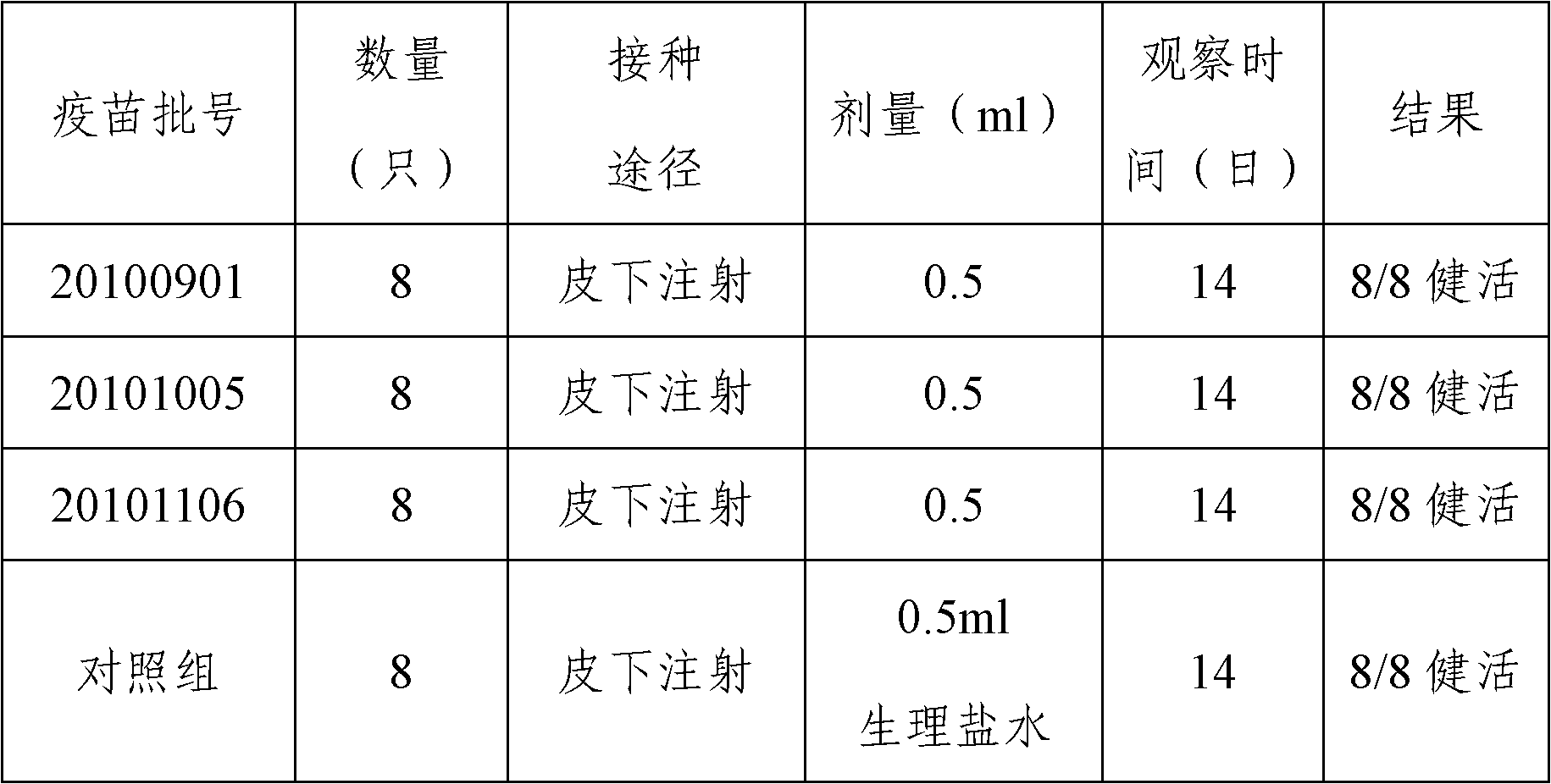
Swine mycoplasma pneumoniae inactivated vaccine and preparation method thereof
A technology for mycoplasma swine pneumonia and inactivated vaccines, which can be applied to bacterial antigen components, respiratory diseases, antibacterial drugs, etc., can solve the problems of difficult promotion and complicated operation of attenuated live vaccines, and achieve good immunogenicity and prevention of mycoplasma hyopneumoniae The effect of disease occurrence and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 swine mycoplasma pneumonia inactivated vaccine
[0047] (1) Bacteria (CGMCC No.4545) liquid preparation
[0048] A. Bacterial culture
[0049] Inoculate Mycoplasma hyopneumoniae CGMCC No.4545 bacterium liquid at a ratio of 10% of the medium volume, and culture it at 37±1°C for 5 days.
[0050] B. Pure Test
[0051] According to the current "Chinese Veterinary Pharmacopoeia" appendix for pure test, should be pure.
[0052] C. CCU determination
[0053] Take the harvested bacterial liquid to inoculate the liquid culture medium, and perform a 10-fold serial dilution to 10 -10 , cultured at 37±1°C for 14 days, the titer of live bacteria was 10 10 CCU / ml.
[0054] D. Concentration and purification
[0055] Centrifuge the bacterial solution at 10,000r / min for 30 minutes, discard the supernatant, suspend the precipitated bacterial cells with PBS (pH7.2-7.4) buffer, prepare a bacterial suspension with 1 / 100 of the original culture volume, ...
Embodiment 2
[0072] The preparation of embodiment 2 swine mycoplasma pneumonia inactivated vaccine
[0073] (1) Bacterial solution preparation
[0074] A. Bacterial culture
[0075] Inoculate the Mycoplasma hyopneumoniae CGMCC No.4545 bacterial solution according to 8% of the medium amount, and culture it at 37±1°C for 9 days, and harvest the bacterial solution when the color of the medium turns yellow and slightly turbid, and the pH value drops to 6.50.
[0076] B. Pure Test
[0077] According to the current "Chinese Veterinary Pharmacopoeia" appendix for pure test, should be pure.
[0078] C. CCU determination
[0079] Take the harvested bacterial liquid to inoculate the liquid culture medium, and perform a 10-fold serial dilution to 10 -10 , cultured at 37±1°C for 14 days, the titer of live bacteria was 10 10 CCU / ml.
[0080] D. Concentrate
[0081] Centrifuge the bacterial solution at 10,000r / min for 30 minutes, discard the supernatant, suspend the precipitated bacterial cells w...
Embodiment 3
[0098] Embodiment 3 preparation of swine mycoplasma pneumonia inactivated vaccine
[0099] (1) Preparation of bacterial solution for seedling production
[0100] A. Bacterial culture
[0101] Inoculate the mycoplasma hyopneumoniae CGMCC No.4545 bacterial liquid according to 9% of the medium amount, and culture it at 37±1°C for 7 days.
[0102] B. Pure Test
[0103] According to the current "Chinese Veterinary Pharmacopoeia" appendix for pure test, should be pure.
[0104] C. CCU determination
[0105] Take the harvested bacterial liquid to inoculate the liquid culture medium, and perform a 10-fold serial dilution to 10 -10 , cultured at 37±1°C for 14 days, the titer of live bacteria was 10 11 CCU / ml.
[0106] D. Concentrate
[0107] Centrifuge the bacterial solution at 10,000 r / min for 30 minutes, remove the supernatant, suspend the precipitated bacterial cells with PBS (pH 7.2-7.4) buffer, prepare a bacterial suspension of 1 / 100 of the original culture volume, and stor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com