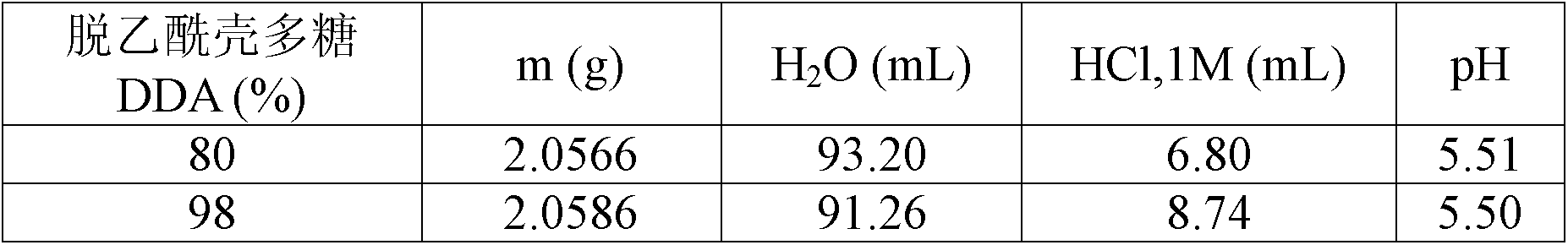Highly biocompatible dual thermogelling chitosan/glucosamine salt compositions
A technology of chitosan and biocompatibility, applied in the field of chitosan solution, can solve problems such as hydrogel that cannot be turned into homogeneous, rise and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0073] Preparation of chitosan-buffer solution mixture
[0074] 1. Preparation of Chitosan Solution
[0075] Chitosan solutions (2.00% w / v) were prepared by dissolving medical grade chitosan with medium molecular weight in aqueous HCl. HCl compared to chitosan amino groups (NH 2 ) ratio (referred to as the degree of protonation of chitosan in solution) was maintained at 70%. This solution was sterilized at 121° C. for 30 minutes using an autoclave. After cooling, water loss caused by the autoclave process is compensated for by adding sterile water in a controlled aseptic environment. The solution was then sterile filtered through a metal frit, dispensed in 5.0 mL aliquots and stored at 4°C. An additional aliquot of approximately 3 mL was used to measure the pH of the chitosan solution. The properties of 100 mL solutions prepared using chitosan with a DDA of approximately 80% and 98% are summarized in Table 1.
[0076] Table 1
[0077] Properties of Chitosan Solution (...
Embodiment II
[0102] Preparation of thermal gelation solutions of chitosan using glucosamine-6-phosphate
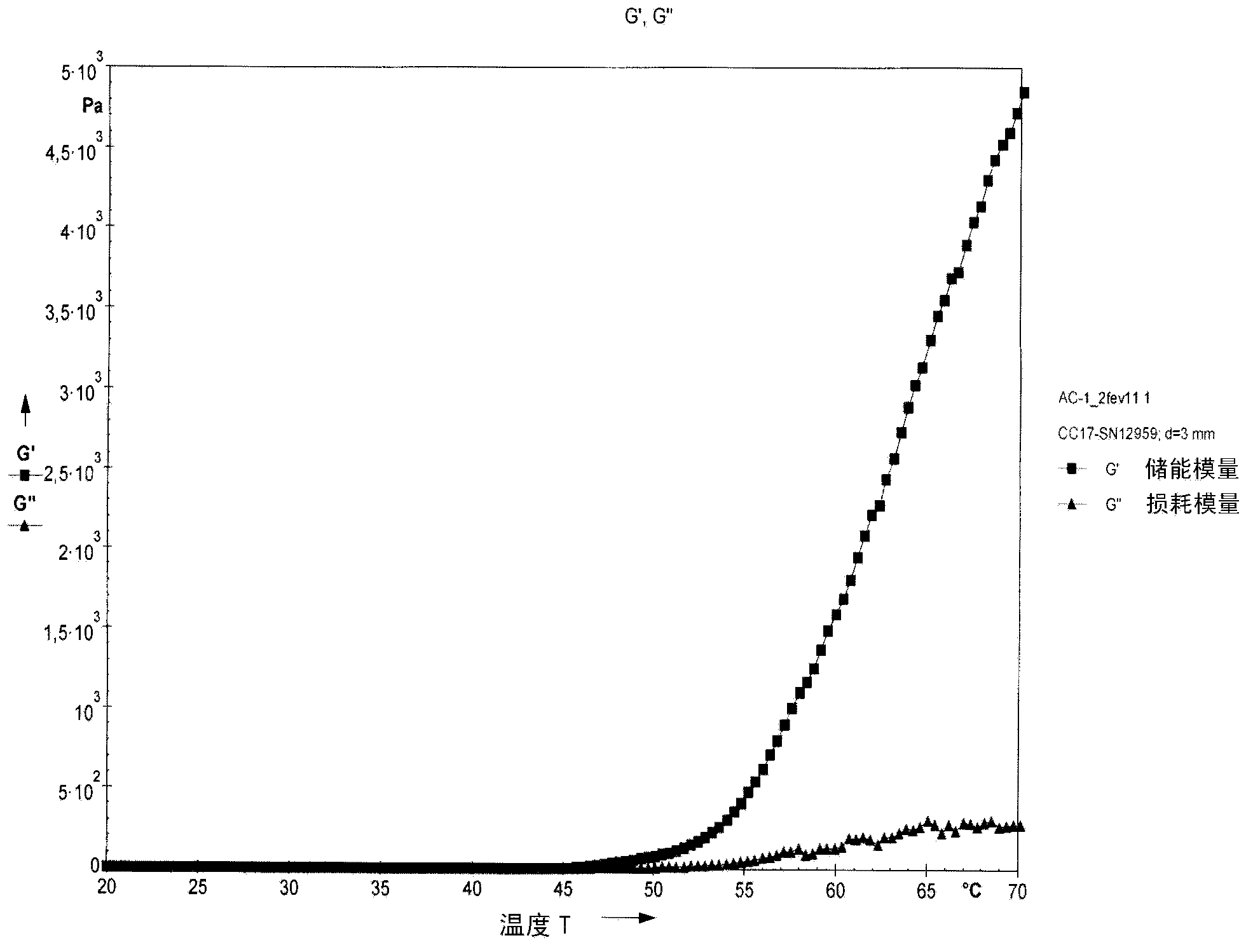
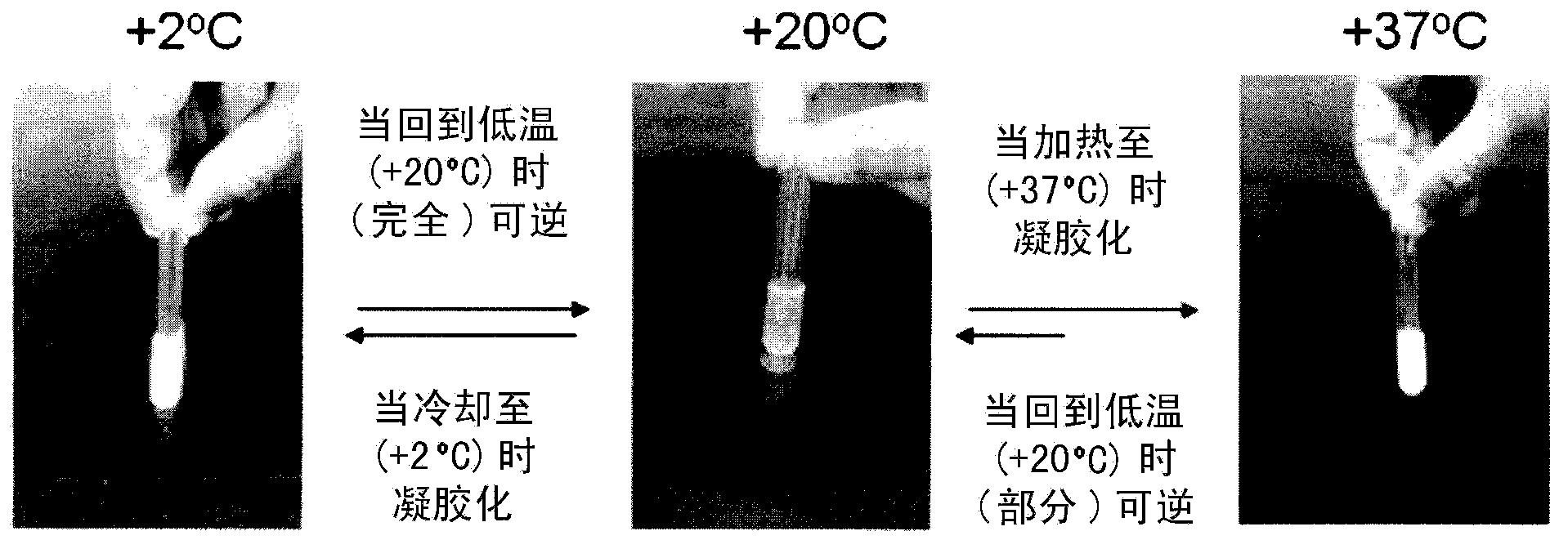
[0103] Chitosan solutions (-2.0% w / v) were prepared as described in Example I above. The thermogelling solution was prepared by mixing 5.0 mL of chilled chitosan solution with 0.5 mL of chilled glucosamine-6-phosphate disodium salt solution (1M) in an ice bath (~4°C) with vigorous stirring. preparation. The resulting solution with a pH of about 7.0 was then removed from the ice bath and placed at 37°C where it gelled within 15 minutes.
Embodiment III
[0105] Therapeutic procedures using dual thermogelling compositions
[0106] The compositions disclosed herein may be used in minimally invasive therapeutic procedures, especially in musculoskeletal tissues such as articular cartilage, fibrocartilage, and bone, to name a few. The compositions described herein are particularly useful for treating articular cartilage damage and have been used clinically in patients with articular cartilage defects. This composition has been used by orthopedic specialists in accordance with clinical protocols and in accordance with Health Canada's Special Access Program (SAP) for the treatment of knees in patients suffering from knee cartilage damage, knee pain and reduced joint function. Articular cartilage defects in joints.
[0107] A total of nine patients have been treated in Canada, aged 18 to 70 years, with intact knee ligament structures and with one-compartment cartilage damage detected by Magnetic Resonance Imaging (MRI). All patients...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of deacetylation | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com