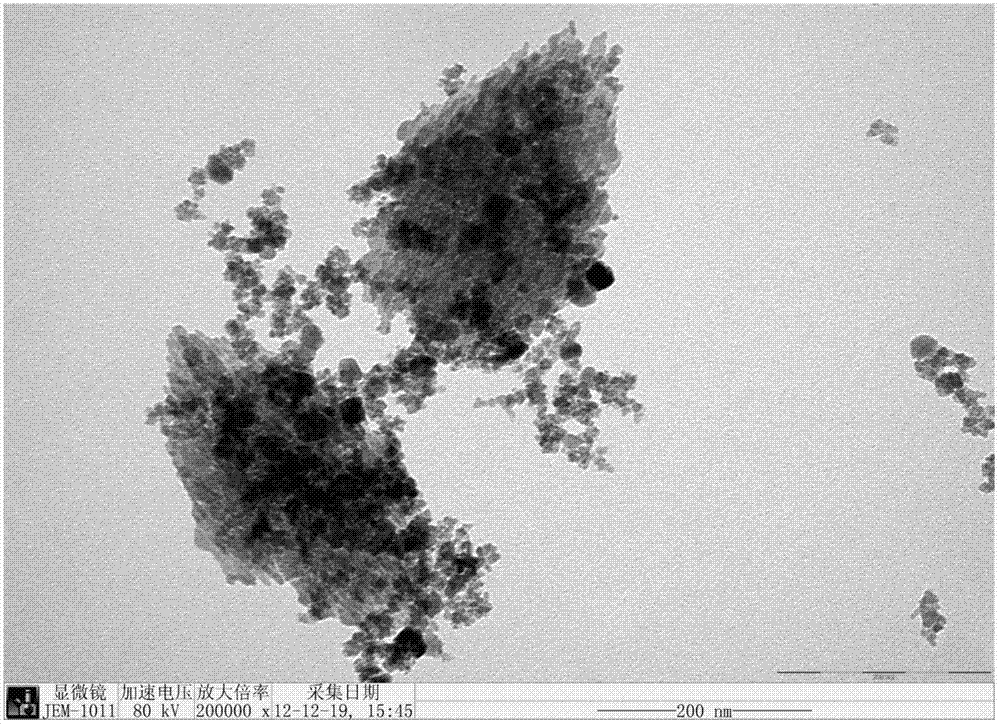
Copper-iron core-shell catalyst for lower alcohol synthesis as well as preparation method and application thereof
A core-shell catalyst and catalyst technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve problems such as the influence of bimetallic core-shell structure that have not been reported, achieve novel structure, prevent Fe sintering, highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 2.42gCu(NO 3 ) 2 ·3H 2 O, 32.32gFe(NO 3 ) 3 ·9H 2 O, 60g urea is dissolved in 1000ml deionized water, the mixed solution is refluxed at 90℃ while mechanically stirring (stirring speed 500rpm), and the pH of the system is continuously monitored until the pH of the solution is 7, and the reflux temperature and stirring speed are unchanged, continue stirring Reflux for 30 minutes, centrifuge, dry at 80°C for 9 hours, and calcinate at 450°C for 2 hours to prepare a catalyst, which is designated as CF-1. The catalyst element molar ratio composition is Cu:Fe 1:8.
[0022] The catalyst is prepared in a pressurized fixed-bed reactor, at 200 ℃ of synthesis gas (molar ratio H 2 / CO=2) Medium reduction, reducing gas volumetric space velocity 3000h -1 , The reduction pressure is 0.1MPa, and the reduction time is 15h. After cooling, the synthesis gas is switched to react. The reactants are collected by a heat trap and a cold trap respectively. The reaction conditions are: raw gas mol...
Embodiment 2
[0024] 2.42gCu(NO 3 ) 2 ·3H 2 O, 16.16gFe(NO 3 ) 3 ·9H 2 O, 60g urea is dissolved in 1000ml deionized water, the mixed solution is refluxed at 90°C while mechanically stirring (stirring speed 500rpm), and the pH of the system is continuously monitored until the pH of the solution is 7, and the reflux temperature and stirring speed are unchanged, continue stirring Reflux for 30 minutes, centrifuge, dry at 80°C for 9 hours, and calcinate at 450°C for 2 hours to prepare a catalyst, which is designated as CF-2. The catalyst element molar ratio composition is Cu:Fe 1:4.
[0025] The catalyst is prepared in a pressurized fixed-bed reactor at 200 ℃ of synthesis gas (molar ratio H 2 / CO=2) Medium reduction, reducing gas volumetric space velocity 3500h -1 , The reduction pressure is 0.1MPa, and the reduction time is 15h. After cooling, the synthesis gas is switched to react. The reactants are collected by heat trap and cold trap respectively. The reaction conditions are: raw gas molar r...
Embodiment 3
[0027] 2.42gCu(NO 3 ) 2 ·3H 2 O, 4.04gFe(NO 3 ) 3 ·9H 2 O, 60g urea is dissolved in 1000ml deionized water, the mixed solution is refluxed at 90°C while mechanically stirring (stirring speed 500rpm), and the pH of the system is continuously monitored until the pH of the solution is 7, and the reflux temperature and stirring speed are unchanged, continue stirring Reflux for 30 minutes, centrifuge, dry at 80°C for 9 hours, and calcinate at 450°C for 2 hours to prepare a catalyst, which is designated as CF-3. The catalyst element molar ratio composition is Cu:Fe 1:1.
[0028] The catalyst is prepared in a pressurized fixed-bed reactor at 200 ℃ of synthesis gas (molar ratio H 2 / CO=2) Medium reduction, reducing gas volumetric space velocity 4000h -1 , The reduction pressure is 0.1MPa, and the reduction time is 15h. After cooling, the synthesis gas is switched to react. The reactants are collected by heat trap and cold trap respectively. The reaction conditions are: raw gas molar ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com