Preparation method of alkanol self-temperature control phase change material
A technology of phase change materials and alkanol, which is applied in the field of preparation of phase change materials and can solve problems such as supercooling and no mention of lowering the phase transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Weigh the sample according to the ratio of paraffin: lauryl alcohol = 1:4, pour it into a beaker, then put it into a thermostat, adjust the temperature, and make the thermostat rise from room temperature to 70 degrees, observe the shape of the sample, and wait until the sample becomes completely transparent When it is in a liquid state, the sample is taken out and cooled at room temperature to obtain the desired product.
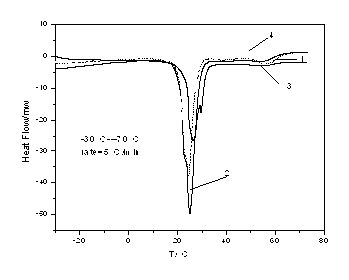
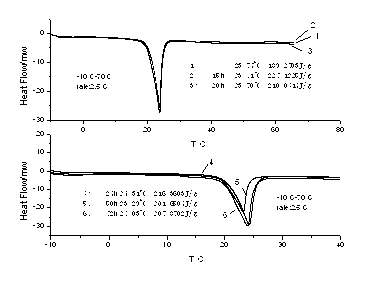
[0020] The phase change temperature of the prepared phase change material is 24.45°C, and the phase change enthalpy is 162.7091 kJ / kg, see attached figure 1 .
Embodiment 2
[0022] Weigh the sample according to the ratio of paraffin: lauryl alcohol = 1:5, pour it into a beaker, then put it into a constant temperature tank, adjust the temperature, and make the temperature of the constant temperature tank rise from room temperature to 70 degrees, observe the shape of the sample, and wait until the sample becomes completely transparent When it is in a liquid state, the sample is taken out and cooled at room temperature to obtain the desired product.
[0023] The phase change temperature of the prepared phase change material is 24.97°C, and the phase change enthalpy is 187.5993 kJ / kg, see attached figure 1 .
Embodiment 3
[0025] Weigh the sample according to the ratio of paraffin: lauryl alcohol = 1:6, pour it into a beaker, then put it into a thermostat, adjust the temperature, and make the thermostat rise from room temperature to 70 degrees, observe the shape of the sample, and wait until the sample becomes completely transparent When it is in a liquid state, the sample is taken out and cooled at room temperature to obtain the desired product. The phase change temperature of the prepared phase change material is 26.38°C, and the phase change enthalpy is 161.04 kJ / kg, see attached figure 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com