Etoposide lyophilized powder for injection
A technology of freeze-dried powder injection and etoposide, which is applied in freeze-dried delivery, powder delivery, medical preparations of inactive ingredients, etc., can solve the problems of low bioavailability and poor water solubility of oral preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Etoposide 80g
[0022] The composition of sorbitol, dextran and lactose (the weight ratio of the three is 1:3:1) 200g
[0023] Composition of arginine and cysteine hydrochloride (the weight ratio of the two is 1:4) 5g
[0024] Disodium edetate 10g
[0025] Hydrochloric acid solution (0.1mol / l) to adjust the pH to 6.5
[0026] Add water for injection to 1000ml
[0027] Preparation:
[0028] Weigh respectively the prescribed amount of etoposide, sorbitol, dextran, lactose, arginine, cysteine hydrochloride and disodium edetate, add to water for injection, fully stir to dissolve, add activated carbon, absorb, Decarburization filter. The hydrochloric acid solution adjusted the pH to 6.5. After filtering with a 0.22μm filter membrane, fill it into washed, dried and sterilized vials, each containing 3mL, half-press the cap, turn on the freeze dryer, cool down to -40°C, freeze-dry, crimp the cap, and check ,Package.
Embodiment 2
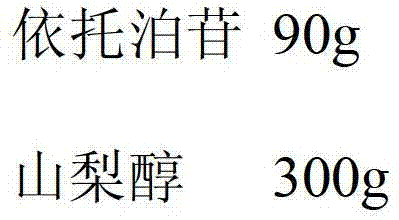
[0030] Etoposide 90g
[0031] Composition of sorbitol, dextran and lactose (the weight ratio of the three is 1:3:1) 300g
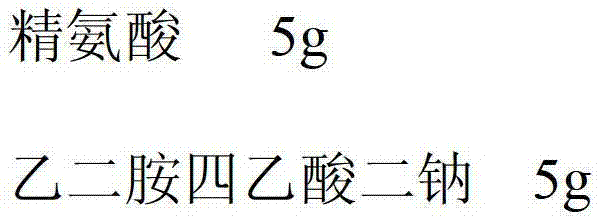
[0032] Composition of arginine and cysteine hydrochloride (the weight ratio of the two is 1:4) 5g
[0033] Disodium EDTA 5g
[0034] Adjust pH to 5.5 with citric acid (0.1mol / l)
[0035] Add water for injection to 1000ml
[0036] Preparation method is the same as embodiment 1
Embodiment 3
[0038]
[0039]
[0040] Adjust pH to 6.0 with citric acid (0.1mol / l)
[0041] Add water for injection to 1000ml
[0042] Preparation method is the same as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com