Binder for electrodes of lithium ion batteries, paste for negative electrodes of lithium ion batteries, and method for producing negative electrode of lithium ion battery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
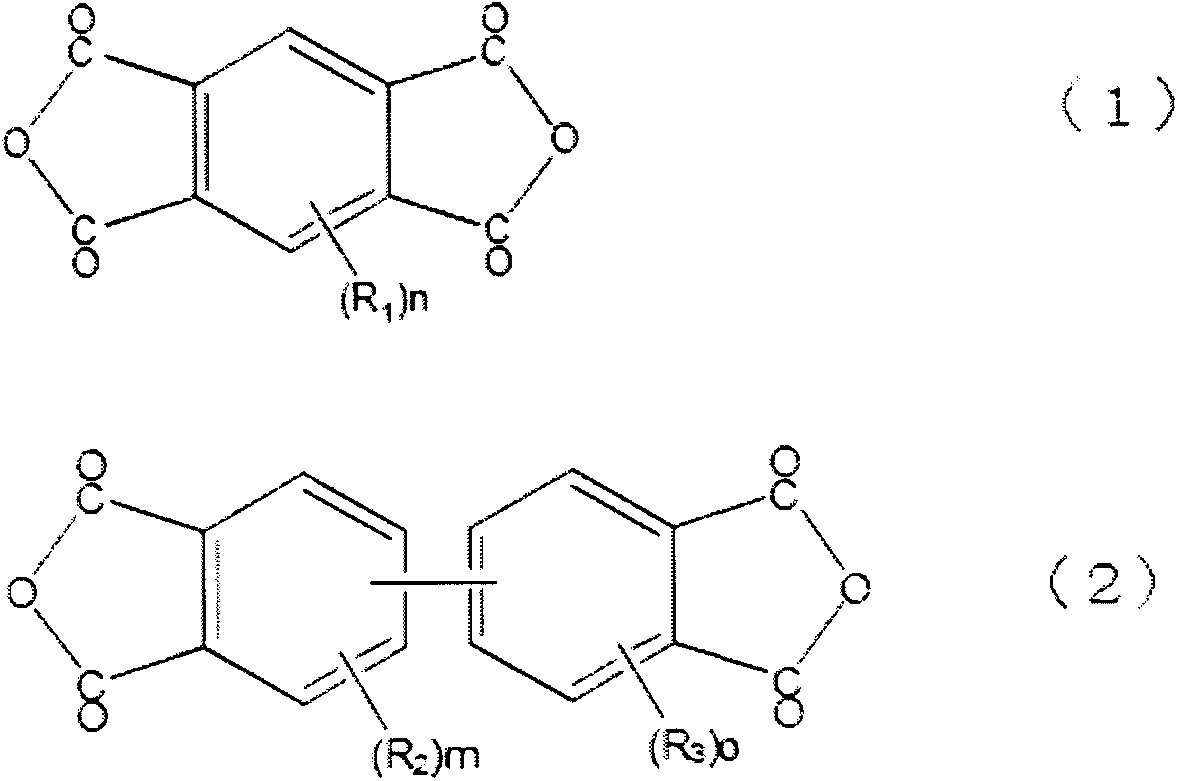
[0022] The tetracarboxylic dianhydride used for preparation of the polyimide precursor used by this invention is selected from the tetracarboxylic dianhydride represented by general formula (1) and (2). As such an example, pyromellitic anhydride, biphenyltetracarboxylic dianhydride, etc. are mentioned. Among them, tetracarboxylic dianhydride represented by general formula (2) is particularly preferable. As a specific example, biphenyl tetracarboxylic dianhydride etc. are mentioned.
[0023] As other tetracarboxylic dianhydrides, benzophenone tetracarboxylic dianhydride, diphenyl ether tetracarboxylic dianhydride, diphenylsulfone tetracarboxylic dianhydride, hexafluoropropylenebis(phthalic anhydride) Wait. Moreover, aliphatic tetracarboxylic dianhydrides, such as cyclobutane tetracarboxylic dianhydride, butane tetracarboxylic dianhydride, cyclopentane tetracarboxylic dianhydride, cyclohexane tetracarboxylic dianhydride, and naphthalene tetracarboxylic dianhydride, can also be...
Embodiment 1
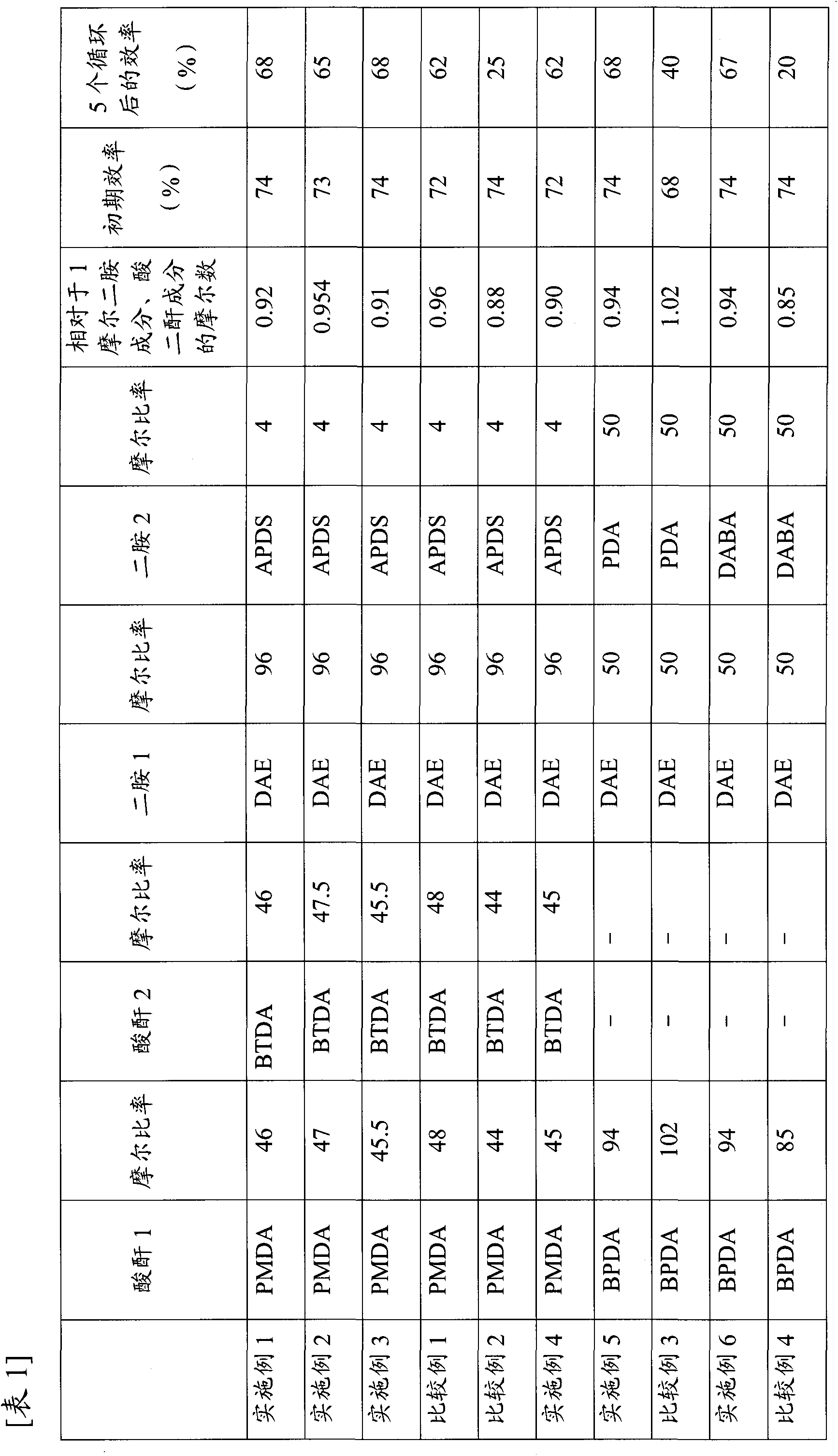
[0075] In a fully dried four-neck separable flask, 19.22 g (96.0 mmol) of DAE and 0.99 g (4.0 mmol) of APDS were dissolved in 150 g of NMP under a nitrogen atmosphere. 10.03 g (46.0 mmol) of PMDA, 14.82 g (46.0 mmol) of BTDA, and 30.2 g of NMP were added thereto, and it stirred while cooling so that it might become 50 degreeC or more. Then, it stirred at 40 degreeC for 4 hours, and obtained the polyamic-acid A solution (20 weight% of solid content concentration).
[0076] 10.8 g of the negative electrode active material produced above was mixed with 6 g of the polyamic acid A solution. The mixture was passed through a three-roll mill 3 times to obtain a negative electrode paste. Using a spatula, the negative electrode paste was applied onto an electrolytic copper foil (manufactured by Nippon Mining Metal Co., Ltd., HLPB) to a thickness of 25 μm. In an inert oven (KOYO THERMO SYSTEMS, INH-9), the electrodeposited copper foil coated with the negative electrode paste was heated...
Embodiment 2
[0079] In a fully dried four-neck separable flask, 19.22 g (96.0 mmol) of DAE and 0.99 g (4.0 mmol) of APDS were dissolved in 150 g of NMP under a nitrogen atmosphere. 10.25 g (47.0 mmol) of PMDA, 15.30 g (47.5 mmol) of BTDA, and 34.0 g of NMP were added thereto, and it stirred while cooling so that it might become 50 degreeC or more. Then, it stirred at 40 degreeC for 4 hours, and obtained the polyamic-acid B solution (20 weight% of solid content concentration).
[0080] A negative electrode was produced in the same manner as in Example 1. The initial efficiency was measured by the above-mentioned method, and it was 73%. The efficiency after 5 cycles was 65%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com