Graphite material, method for producing same, carbon material for battery electrodes, and battery
A technology of graphite materials and manufacturing methods, applied in battery electrodes, graphite, secondary batteries, etc., can solve problems such as poor cycle characteristics, low initial efficiency, and reduced electrode performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
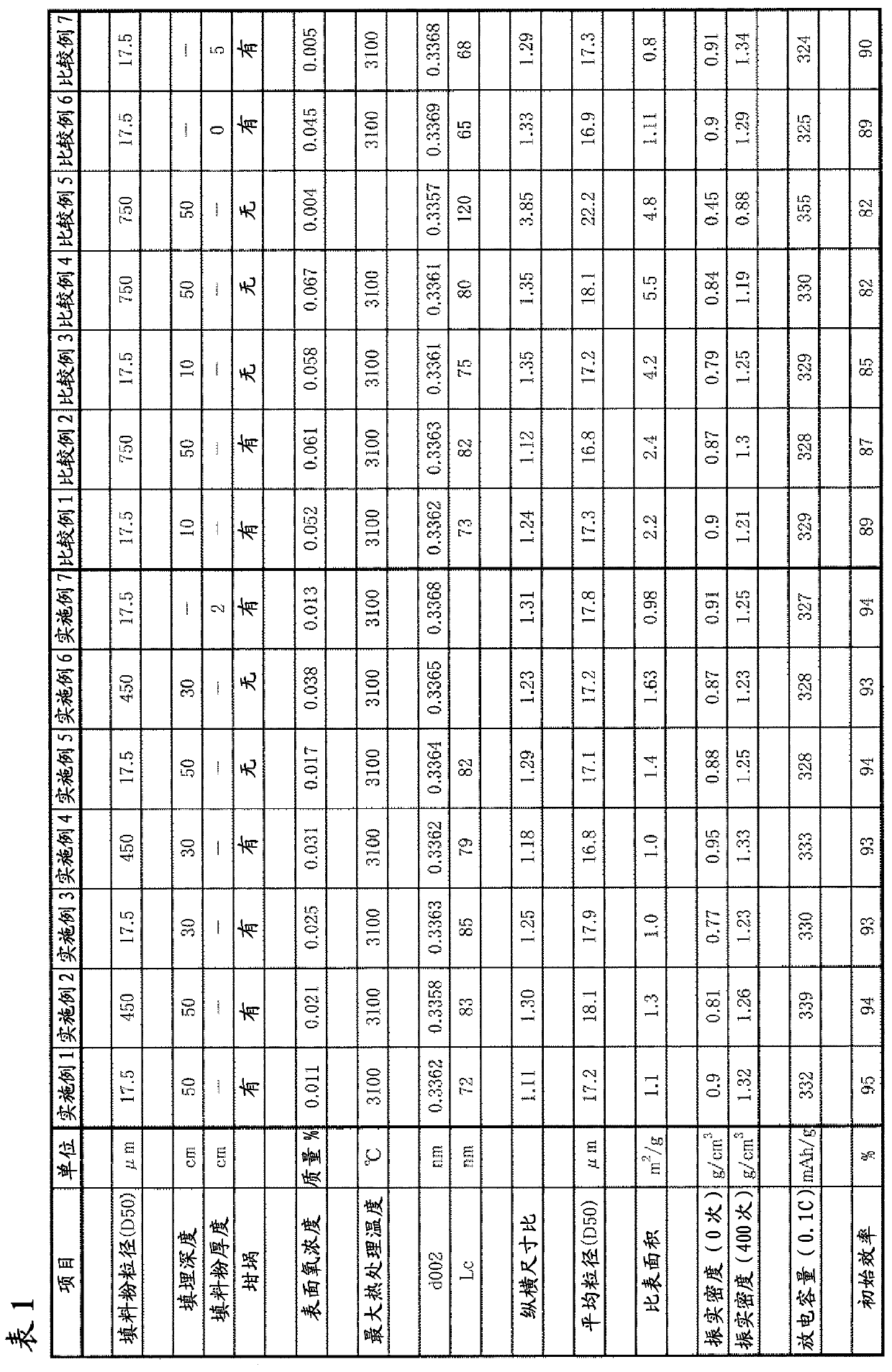
[0171]The residue after vacuum distillation of crude oil produced in Venezuela (specific gravity 3.4° API, asphaltene content 21%, resin content 11%, sulfur content 3.3%) is used as raw material and put into the delayed coking process. At this time, the operation was performed with the outlet temperature of the heating furnace heater in front of the coke drum set at 570°C. The internal pressure is 20 psig. Next, water-cooled and discharged from the coke drum, then heated at 120° C., and dried until the water content was 0.5% or less, was used as dry coke 1 . At this point, the heating loss of coke in the argon atmosphere from 300° C. to 1200° C. was 11.8% by mass. This was pulverized with a bantam mill manufactured by Hosokawa Micron. Next, airflow classification was performed with a turbo classifier TC-15N manufactured by Nisshin Engineering to obtain a carbon material with D50=17.5 μm. The pulverized carbon material was filled in a graphite crucible with a screw cap under...
Embodiment 2
[0174] Dried coke 1 used in Example 1 was adjusted with a small mill and a turbo classifier so that D50 became 17.5 μm. This pulverized carbon material was filled into a graphite crucible with a screw cap.
[0175] Next, in the Acheson furnace for graphitizing artificial graphite electrodes, the filler powder 2 was filled with a carbon material (aspect ratio of 2.5) obtained by pulverizing petroleum-based coke 1 into a D50 of 450 μm. The above-mentioned graphite crucible was buried in the Acheson furnace to a depth of 50 cm. The distance from the bottom of the Acheson furnace to the bottom of the graphite crucible is 50cm, and the shortest distance from the side of the Acheson furnace to the side of the graphite crucible is 50cm. The furnace was energized and heat-treated at 3100° C. to obtain a graphite material. After measuring various physical properties of this sample, electrodes were fabricated as described above, and cycle characteristics and the like were measured. T...
Embodiment 3
[0177] Dried coke 1 used in Example 1 was adjusted with a small mill and a turbo classifier so that D50 became 17.5 μm. This pulverized carbon material was filled into a graphite crucible with a screw cap.
[0178] Next, in the Acheson furnace for graphitizing artificial graphite electrodes, the carbon material obtained by pulverizing petroleum-based coke 1 to a D50 of 17.5 μm was filled as filler powder 1 . The aforementioned graphite crucible was embedded in the Acheson furnace to a depth of 30 cm. The distance from the bottom of the Acheson furnace to the bottom of the graphite crucible is 50cm, and the shortest distance from the side of the Acheson furnace to the side of the graphite crucible is 50cm. The furnace was energized and heat-treated at 3100° C. to obtain a graphite material. After measuring various physical properties of this sample, electrodes were fabricated as described above, and cycle characteristics and the like were measured. The results are shown in T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com