Buffered ophthalmic compositions and methods of use thereof
An ophthalmic composition and composition technology, applied in the direction of drug combination, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of not enhancing wound closure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
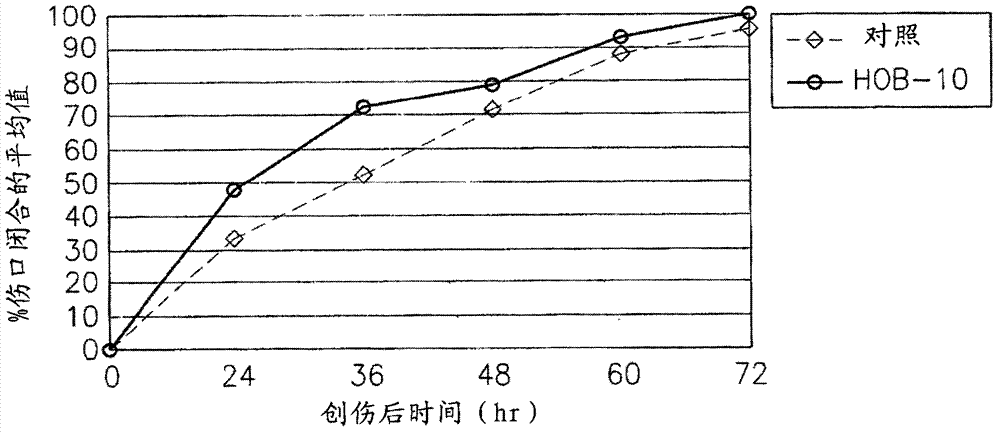
[0152] Therapeutic Effects of Ophthalmic Buffers
[0153] Formulation 1 was formulated for eye treatment and to potentiate the action of MPDY-1. In order to determine the therapeutic effect of Ophthalmic Buffer Formulation 1 on corneal ulcers and ocular conditions, a series of experiments were performed with rabbit eyes. Corneal ulcers were induced in rabbit eyes by mechanical or chemical means and subsequently treated with formulation 1 buffer solution or control. Corneal ulcers are induced as follows:
[0154] Mechanical corneal ulceration: 6 mm corneal trephine (Grieshaber, Switzerland), preset to 50 microns deep, and a uniform central corneal epithelial erosion, 6 mm in diameter and 50 microns deep, using a mini-blade under an operating microscope.
[0155]Chemical Alkaline Ulceration: 5 mm absorbent paper disks were used to perform corneal alkaline erosions by placing 120 μl of NaOH 1 N for 10 seconds. Eyes were rinsed thoroughly with sterile irrigation water until t...
Embodiment 2
[0170] Therapeutic Effects of MPDY-1 Alone and in Combination with Additional Pharmaceutical Activities
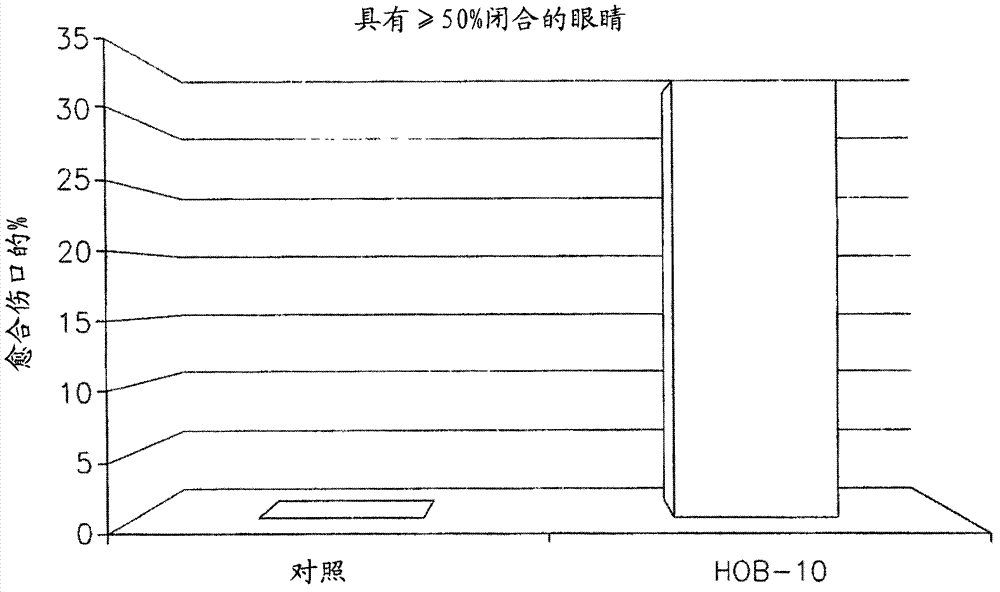
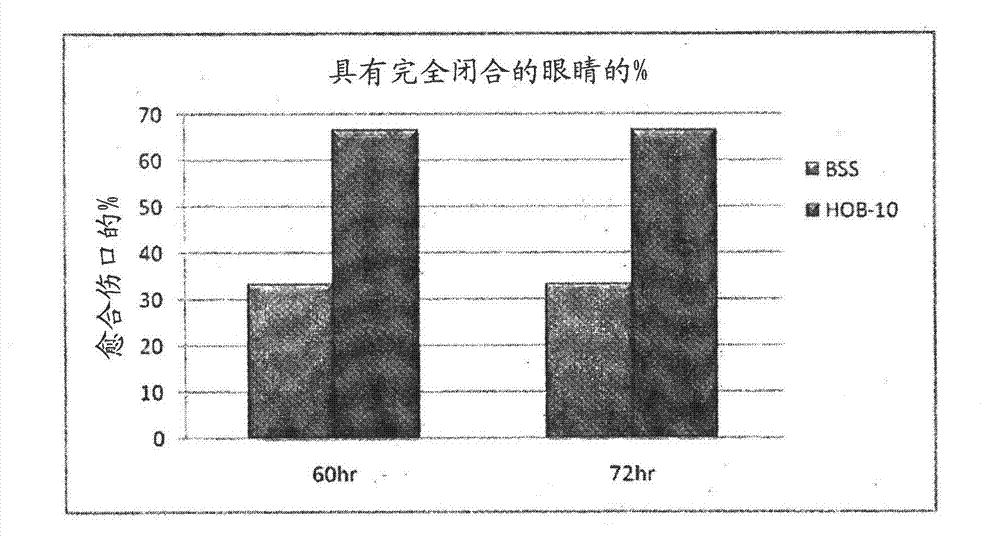
[0171] This example discussion shows that soluble in BSS or DPBS - / - (referred to as formulation 2) of MPDY-1 and dissolved in DPBS with insulin - / - Experiment of the therapeutic effect of MPDY-1 (referred to as formulation 3) or MPDY-1 dissolved in HOB-10 (referred to as HO / 05 / 09) on mechanical and chemical corneal trauma.
[0172] Rabbits were subjected to mechanical corneal trauma as discussed in Example 1. Eyes were treated with twice-daily ocular instillation treatments for 3 consecutive days. Corneal erosion was measured at six-hour intervals using fluorescent staining. Figure 8 Display with separate vehicle (DPBS - / - , left), formulation 3 (MPDY-1 0.1 μg / eye / treatment and insulin 0.01 μg / eye / treatment, (center) and formulation 2 (MPDY-1 0.1 μg / eye / treatment, right) with completely closed Comparison of the percentage of eyes. Formulation 3 and Formulation 2 c...
Embodiment 3
[0187] Anti-inflammatory effect of MPDY-1
[0188] This example discusses experiments showing the anti-inflammatory effects of MPDY-1. The anti-inflammatory effect of MPDY-1 was confirmed using different in vitro, ex vivo and in vivo models. MPDY-1 was shown to attenuate ICAM-1 expression on epithelial cells and keratinocytes, inhibit the infiltration of macrophages, neutrophils and T cells into inflammatory sites and attenuate the activity of macrophages at inflammatory sites.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com