Methanation reaction system and regeneration technology of methanation catalyst
A reaction system, methanation technology, applied in the field of methanation reaction system and its technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
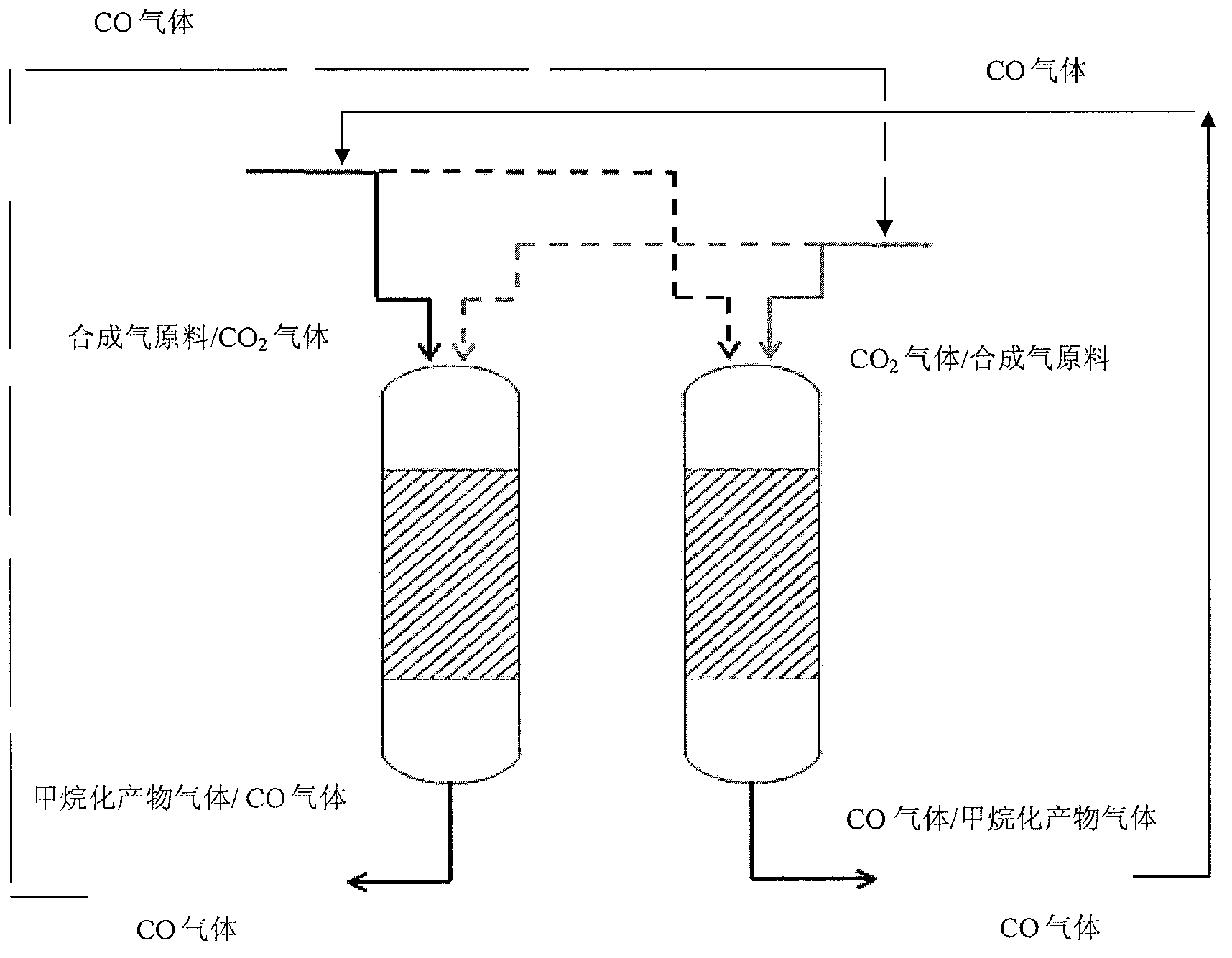
[0047] use as figure 2 The shown methanation reaction system and process of the present invention are used for methanation reaction experiments.
[0048] The chemical composition of the catalyst used is: 15NiO / 100ZrO 2 (weight ratio).
[0049] The methanation reaction conditions are: reaction temperature: 450°C, reaction pressure: 0.1MPa, reaction time: 5 hours, H in the synthesis gas raw material 2 / CO (volume ratio) is 1, the syngas feed temperature is 100°C, and the space velocity (GHSV) of the syngas at the reactor inlet is 24,000 times the catalyst volume per hour.
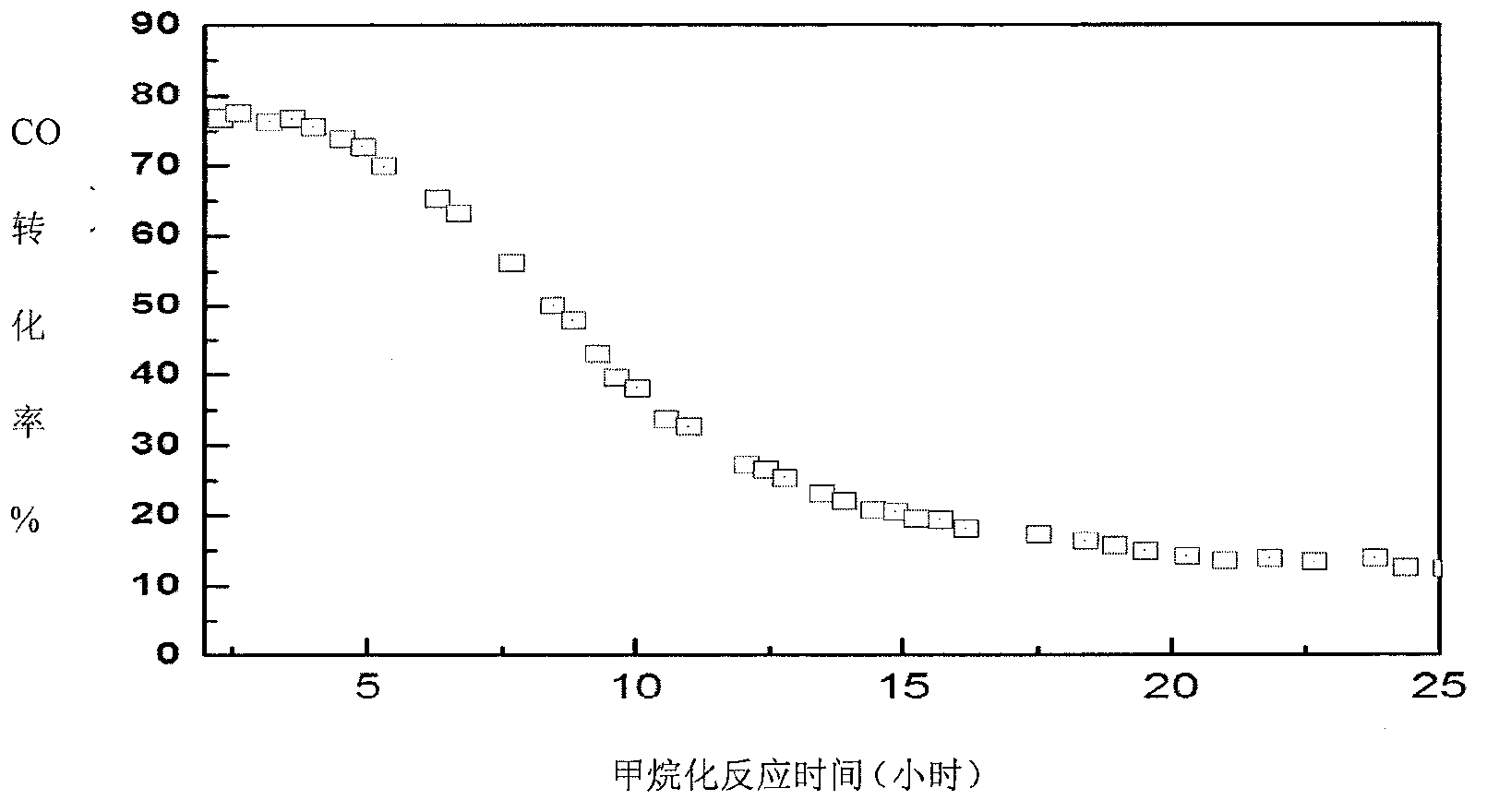
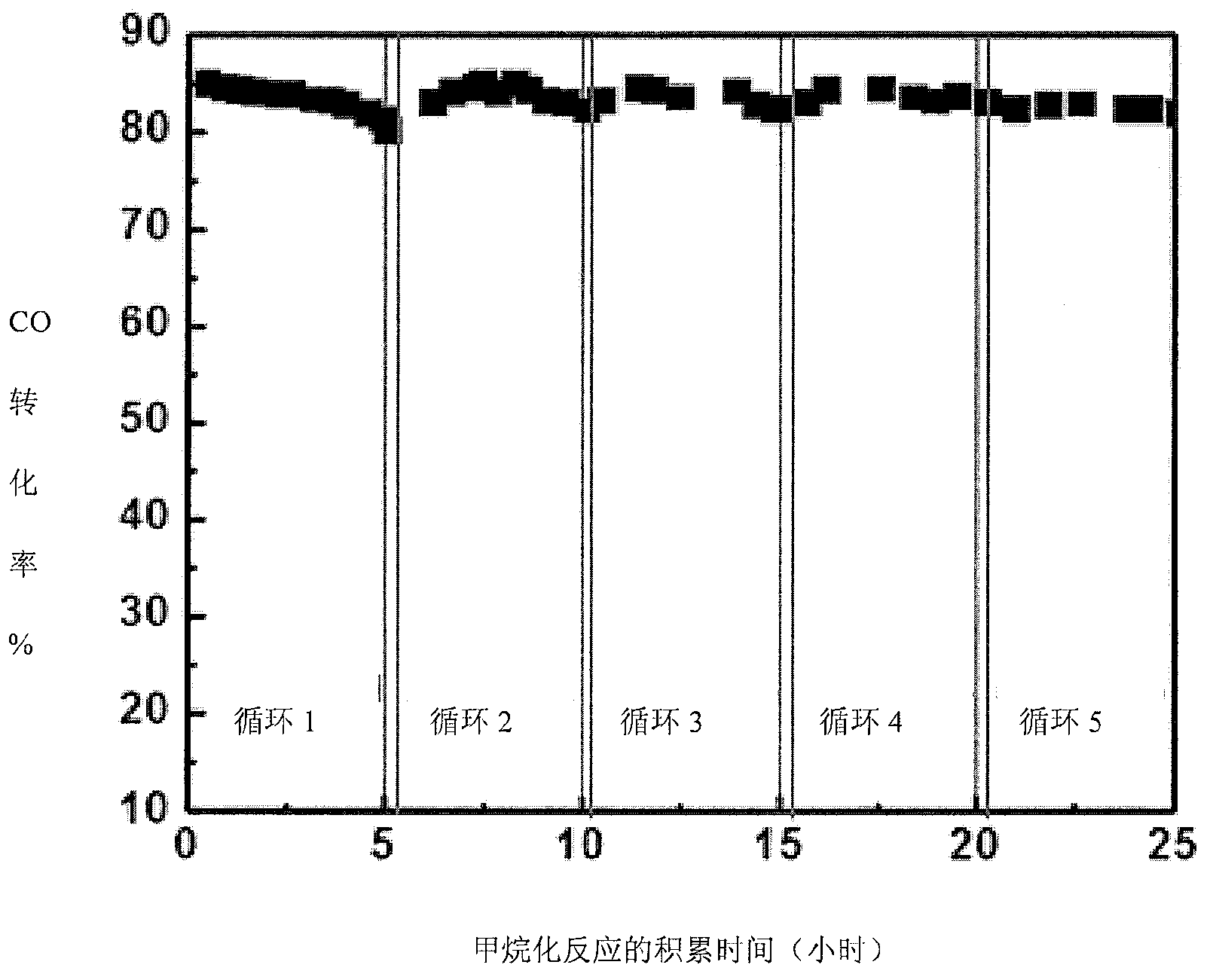
[0050] After 5 hours of reaction, the CO conversion rate dropped from 85% to 80%, indicating that the Ni-based catalyst had carbon deposition. At this time, the feed gas is composed of H 2 and CO syngas feedstock switching to CO 2 The raw material gas makes the carbon-deactivated catalyst in the reactor undergo carbon elimination regeneration reaction, and at the same time feeds the synthetic gas raw ma...
Embodiment 2
[0059] In addition to changing the catalyst chemical composition from 15NiO / 100ZrO 2 (weight ratio) becomes 20NiO / 100ZrO 2 (weight ratio), change the methanation reaction pressure and carbon removal regeneration reaction pressure from 0.1MPa to 3MPa, change the methanation reaction time from 5 hours to 3 hours, and change the number of methanation reaction-carbon removal regeneration reaction cycles from 5 Times become 10 times, repeat the experimental process of embodiment 1.
[0060] The experiment found that the original CO conversion rate of the catalyst was 98%, and the CO conversion rate of the catalyst during carbon removal and regeneration was 90%. After each methanation reaction-carbon removal regeneration reaction cycle, the catalyst CO conversion rate (catalytic activity) was basically Return to the original level, that is, the CO conversion rate of the catalyst is restored from 90% to 98%.
Embodiment 3
[0062] In addition to changing the catalyst chemical composition from 15NiO / 100ZrO 2 (weight ratio) becomes 18NiO / 100ZrO 2 (weight ratio), the H in the syngas 2 / CO (volume ratio) was changed from 1 to 2, and the methanation reaction time was changed from 5 hours to 15 hours, the experimental process of Example 1 was repeated.
[0063] The experiment found that the original CO conversion rate of the catalyst was 95%, and the CO conversion rate of the catalyst during carbon removal and regeneration was 87%. Return to the original level, that is, the CO conversion rate of the catalyst is restored from 87% to 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com