Support for affinity chromatography and method for isolating immunoglobulin
一种免疫球蛋白、载体的技术,应用在亲和色谱用载体和分离免疫球蛋白领域,能够解决容量降低、碱性条件不稳定等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0093] 2. Example
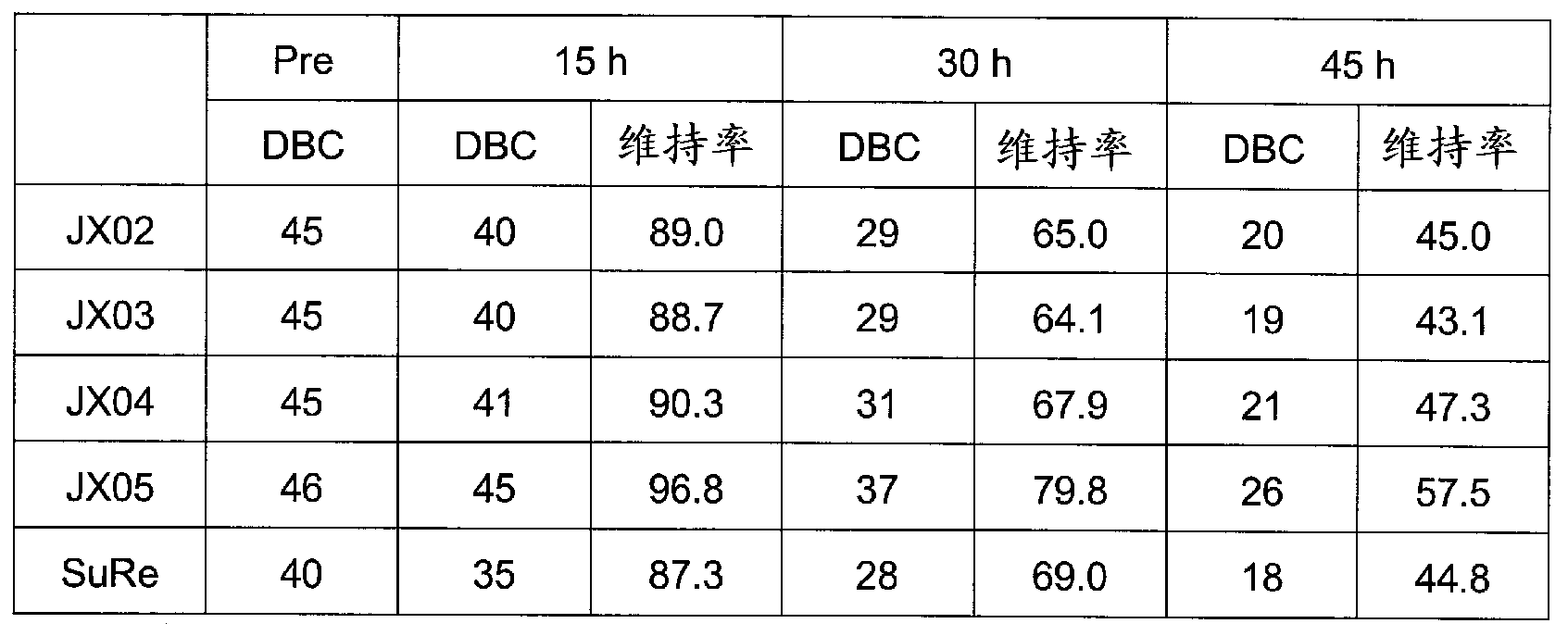
[0094] Hereinafter, the filler for affinity chromatography according to the present embodiment will be described more concretely with reference to examples. In addition, the following description generally shows the form of this invention, and there is no particular reason, and this invention is not limited to the said description.
manufacture example 1
[0095] 2.1. Production Example 1 (Synthesis of Porous Particles)
[0096] Dissolve 8.2 g of glycidyl methacrylate (manufactured by MITSUBISHI RAYON), 65.9 g of trimethylolpropane trimethacrylate (manufactured by Sartomer), and 90.6 g of glyceryl monomethacrylate (manufactured by NOF Corporation) Add 2,2'-azobisisobutyronitrile (manufactured by Wako Pure Chemical Industries, Ltd.) to 245.8 g of 2-octanone (manufactured by Toyo Gosei Kogyo Co., Ltd.) and 62 g of acetophenone (manufactured by Wako Pure Chemical Industries, Ltd.) , to prepare an organic monomer solution.
[0097] Next, 8.5 g of polyvinyl alcohol (PVA-217 manufactured by Kuraray), 0.43 g of sodium lauryl sulfate (Emal10G manufactured by Kao Corporation), and 21.3 g of sodium sulfate (manufactured by Wako Pure Chemical Industries, Ltd.) were added to 4240 g of pure water. g, stirred overnight to prepare an aqueous solution.
[0098] Next, the obtained aqueous solution was poured into a 7L separable flask, a thermome...
manufacture example 2
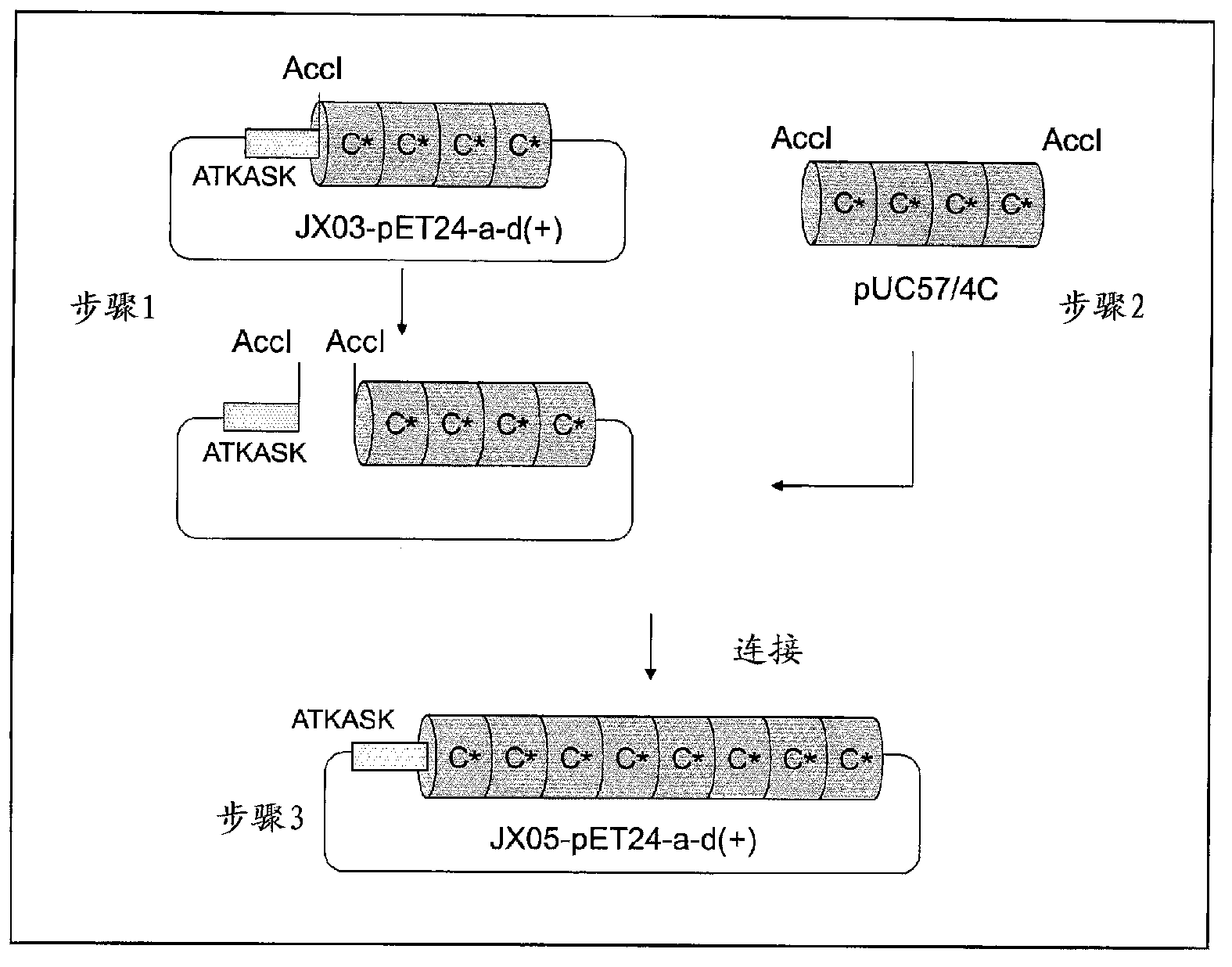
[0100] 2.2. Production example 2 (production of recombinant immunoglobulin-binding protein (JX01))
[0101] In this production example and the following production examples, GE Healthcare's PCR purification kit (PCR Purification Kit) was used for purification of DNA. Restriction enzymes were purchased from New England Biolabs. Enzymes for PCR were purchased from Fermentas. Primers for PCR were purchased from Sigma. Ligation was performed using T4 DNA ligase (ligase) (Invitrogen). DH5α cells were purchased from Invitrogen. BL21 cells were purchased from STRATAGENE. pET24a-d(+) were purchased from Novagen. Unless otherwise specified, all experiments were performed under the conditions recommended by the Supplier.
[0102] 2.2.1. Construction of JX01 plasmid (JX01-pETM11)
[0103] Plasmid JX01-pETM11 containing DNA (SEQ ID NO: 3) encoding the C* domain of the A1V G29A variant, which is the C domain of protein A, was constructed according to the following procedure.
[010...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com