Marbofloxacin freeze-dried powder for injection and preparation method and application thereof
A technology of Mabaofloxacin and freeze-dried powder, which is applied in the field of Mabaofloxacin freeze-dried powder for injection and its preparation, to achieve the effects of convenient use, overcoming insolubility in water, and accurate dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
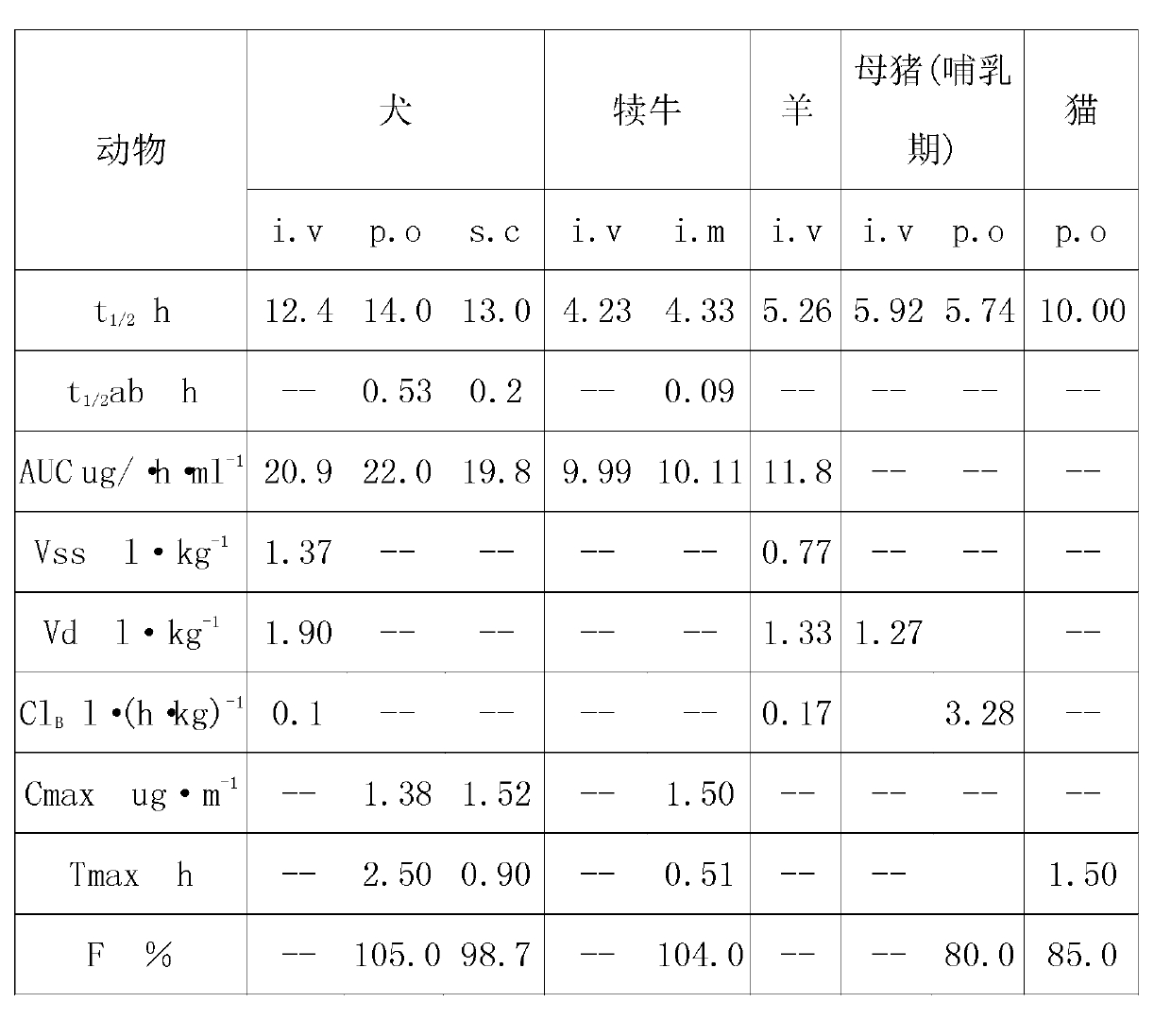
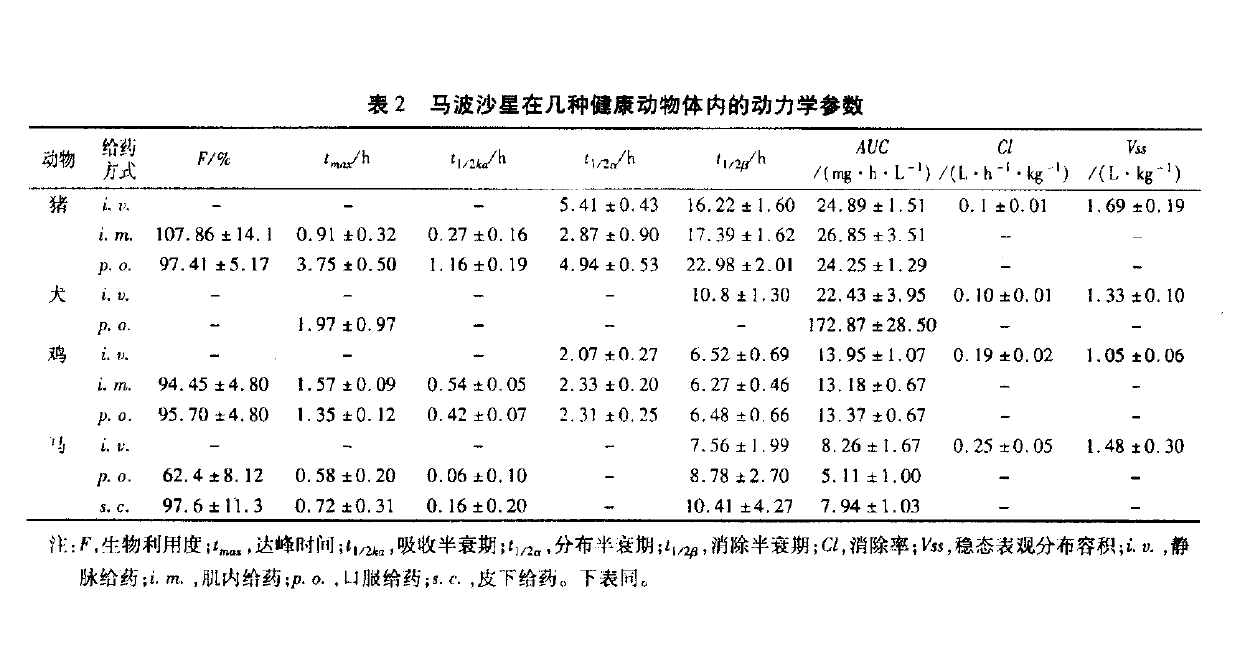
Image
Examples
Embodiment 1
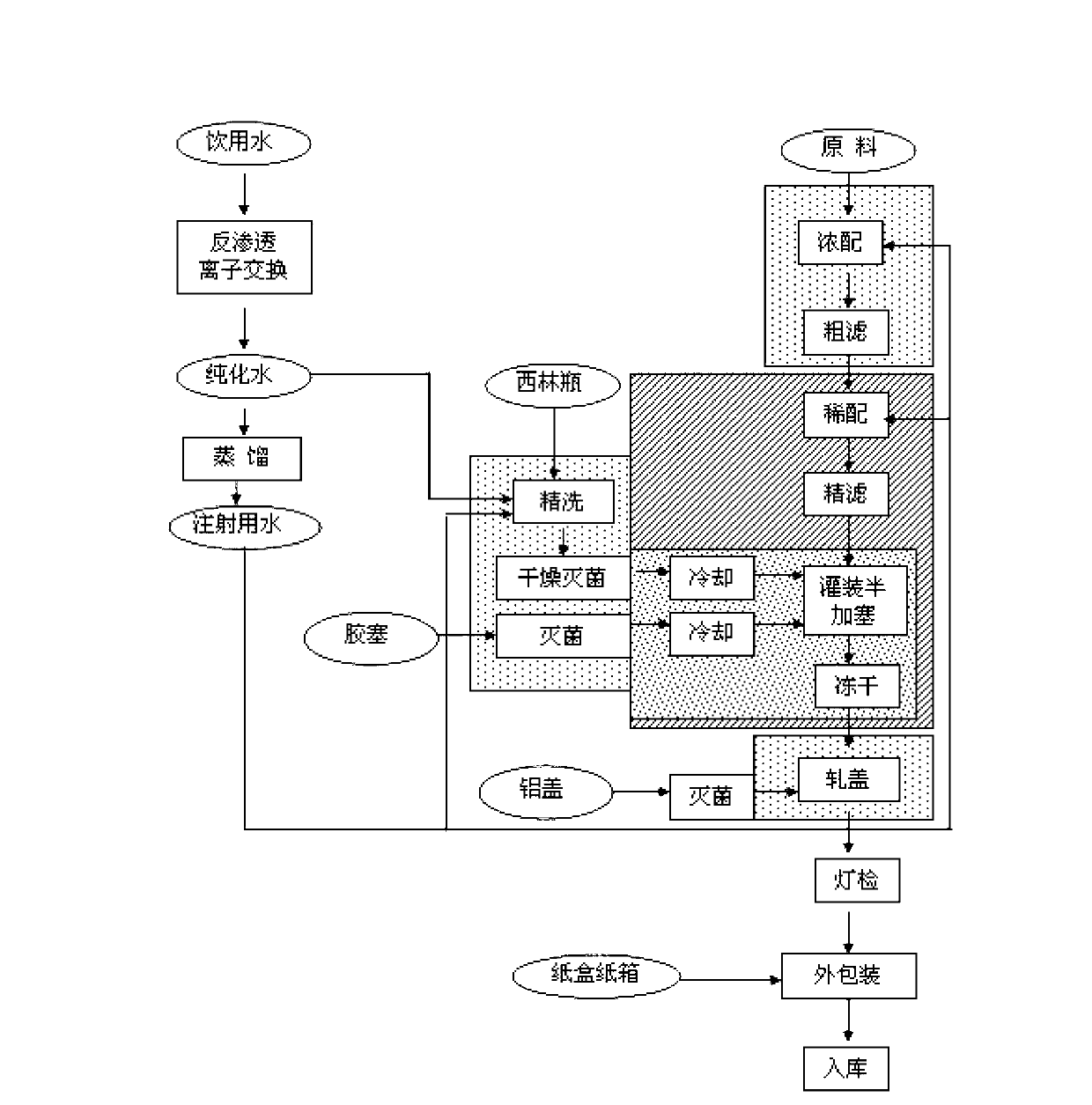
[0074] A freeze-dried powder of Mabaofloxacin for injection, consisting of the following components:
[0075] Mabaofloxacin: 250g; Zhejiang Guobang Pharmaceutical Co., Ltd., production batch number: 090705 Lactic acid (analytical pure): appropriate amount; Shanghai Yanchen Chemical Co., Ltd., batch number: 20100611 Activated carbon for injection: 4.8g; Shandong Xintong activated carbon filter material Co., Ltd. Lot number: 20100812
[0076] Add water for injection to: 5000ml;
[0077] Wherein, the addition amount of lactic acid is to adjust the pH of the preparation to be 4.8-4.9.
[0078] The preparation process of described marbofloxacin freeze-dried powder for injection comprises the following technical steps:
[0079] (1) Preparation of water for injection:
[0080] (a) Purified water is obtained by subjecting drinking water to reverse osmosis ion exchange;
[0081] (b) Purified water can be distilled to obtain water for injection;
[0082] (2) Sterilization of rubber...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com