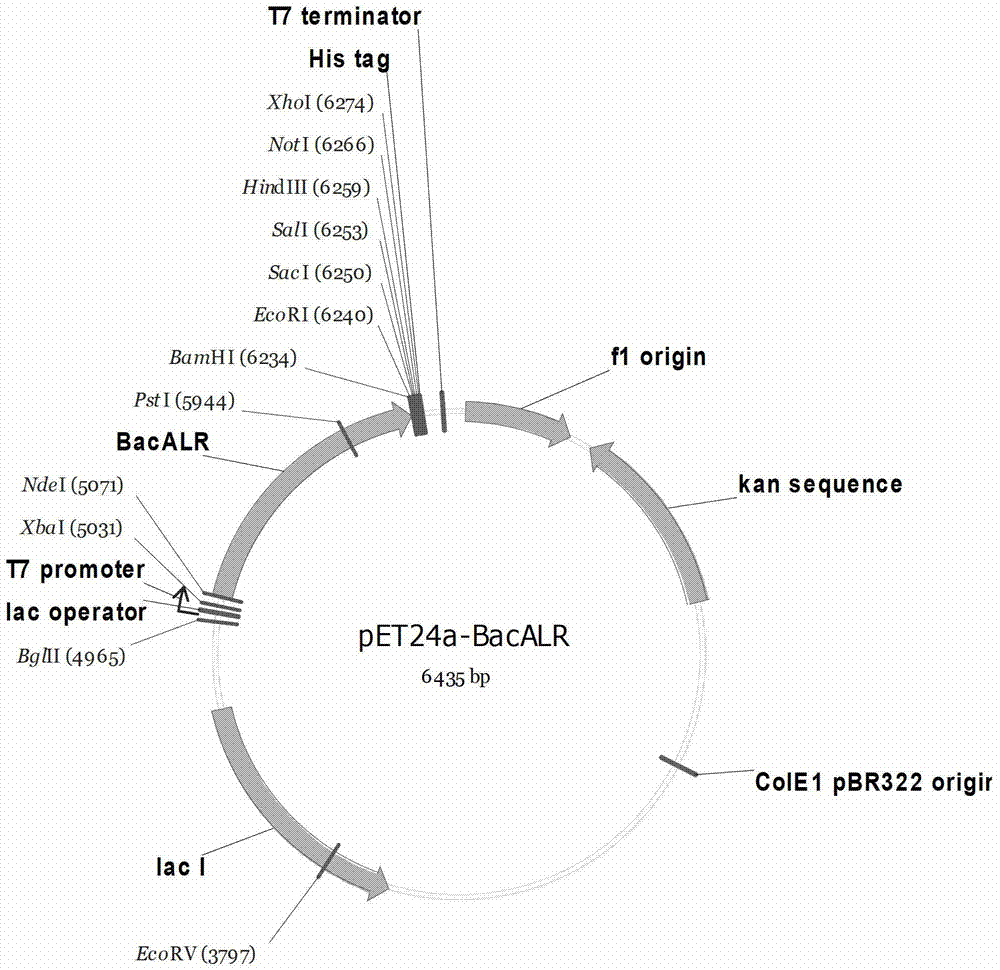
High-yield dl-alanine production strain and its application
A technology of alanine and alanine racemase, applied in the direction of bacteria, microorganisms, recombinant DNA technology, etc., can solve the problem of lack of efficient production of alanine racemase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] The expression strain provided by the present invention can be efficiently applied to the production of DL-alanine. In a preferred embodiment of the present invention, the preparation method of DL-alanine includes the steps:
[0087] Use the strain provided by the invention to transform the raw material L-alanine to obtain DL-alanine;
[0088] Or the method includes: using the fermentation broth of the strain provided in the present invention to transform the raw material L-alanine to obtain DL-alanine. Wherein, the fermentation broth is a fermentation broth containing bacteria.
[0089] In another preferred embodiment, the method includes: culturing the strain of the present invention in the presence of L-alanine.
[0090] In another preferred embodiment, the conversion rate of L-alanine is ≥95%, preferably ≥98%, and more preferably ≥99%.
[0091] In another preferred example, the amount of the strain is 0.5-5 g / l, preferably 1-3 g / l.
[0092] In another preferred example, the ...
Embodiment 1
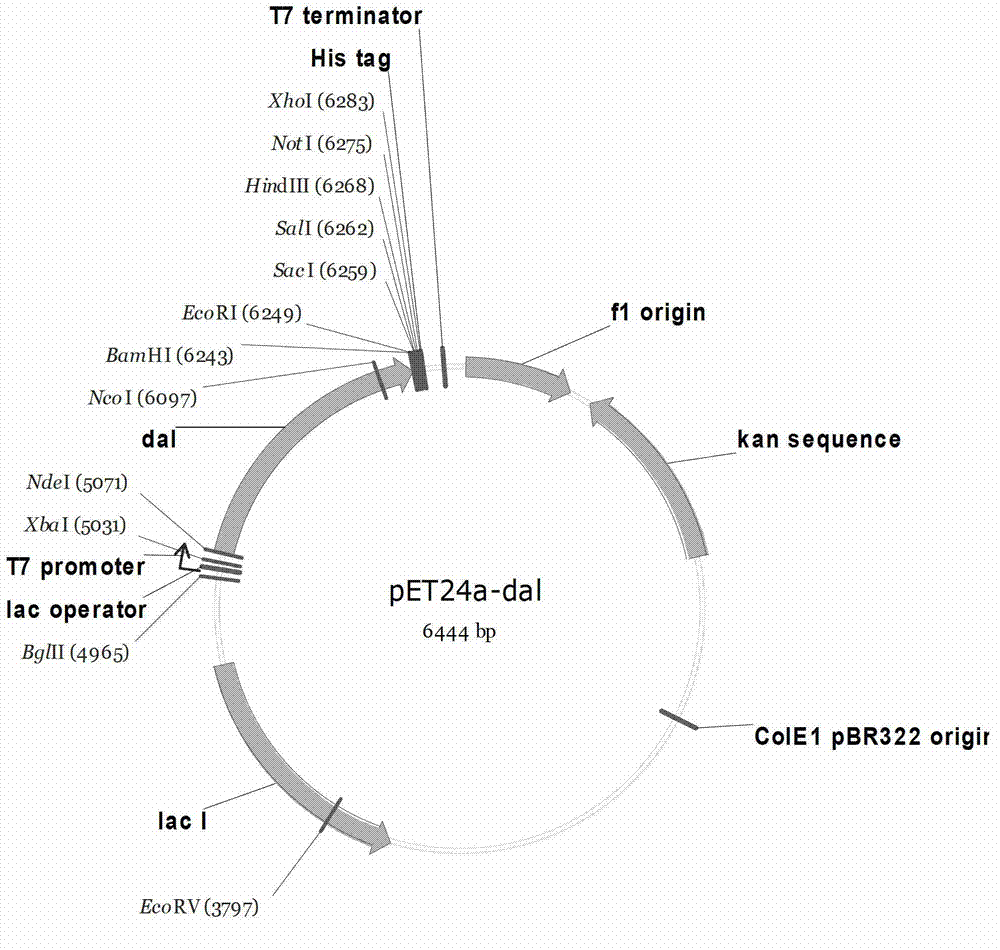
[0118] Example 1 Construction of strain expressing D-alanine racemase from Bacillus subtilis
[0119] Bacillus subtilis subsp. subtilis str. 168 was inoculated into LB liquid medium and cultured at 30°C at 220 rpm for 24 hours. The extraction of total DNA refers to the instructions of the genome extraction kit.
[0120] According to the reported gene sequence of the D-alanine racemase gene dal from Bacillus subtilis subsp.subtilis str.168 (NCBI accession number: AL009126.3), the sense primer and the antisense primer were synthesized. The nucleotide sequences of the primers are recorded in SEQ ID NO: 1 (dal-NdeI-F) and SEQ ID NO: 2 (dal-BamHI-R), respectively.
[0121] SEQ ID NO: 1dal-NdeI-F
[0122] GGAATTCCATATGAGCACAAAACCTTTTTAC
[0123] SEQ ID NO: 2dal-BamHI-R
[0124] CGGGATCCTTAATTGCTTATATTTACCT
[0125] Perform PCR amplification on the reaction solution. 50μL of the reaction solution contains the above-mentioned pair of primers. Each primer is 50pmol, 0.2mM dNTP, 100ng total DNA t...
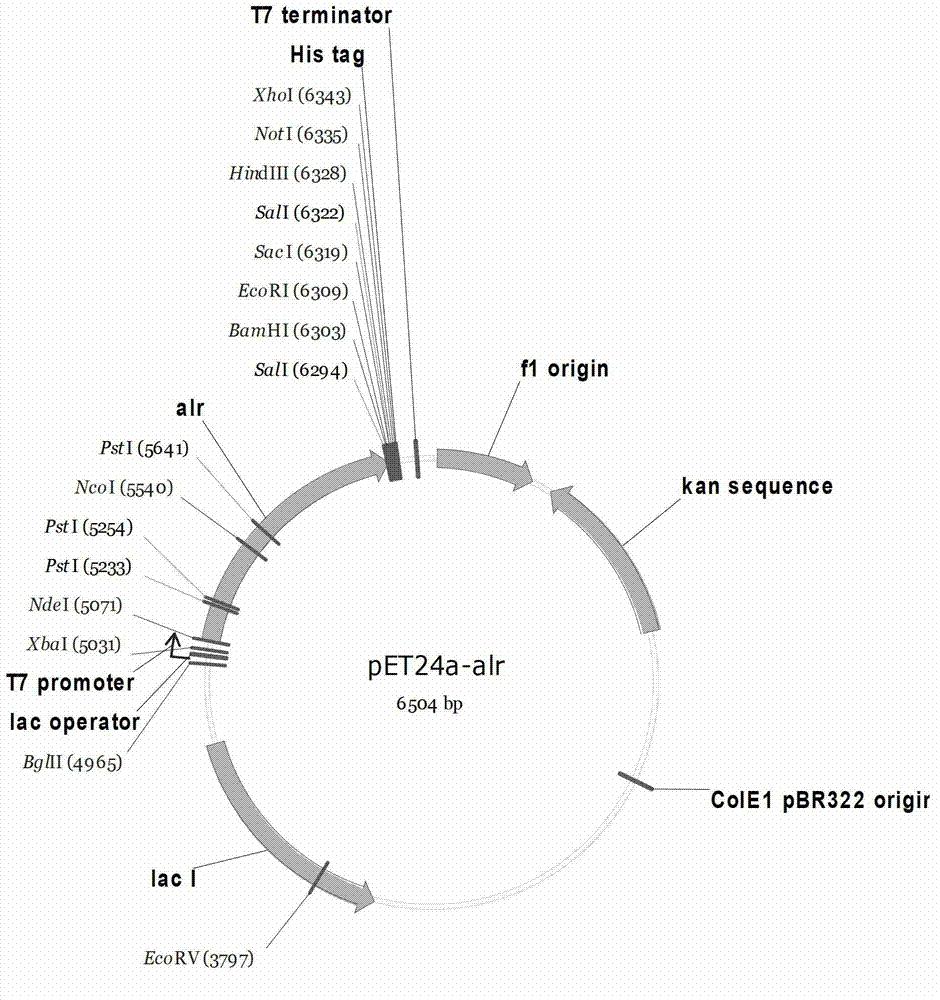
Embodiment 2
[0128] Example 2 Construction of alanine racemase-expressing strains derived from Pseudomonas putida KT2440
[0129] The alanine racemase gene alr (NCBI accession number: NC_002947) derived from Pseudomonas putida KT2440 was fully synthesized to obtain the recombinant plasmid pUC57-alr.
[0130] The pUC57-alr was double digested with NdeI and BamHI at 37°C for 3-6 hours. The digestion system was: pUC57-alr 38 μL, 10X Buffer Tango 10 μL, NdeI 1 μL, BamHI 1 μL, nucleic acid electrophoresis, and a gel recovery kit to recover 1.2 kb alr fragments.
[0131] The recovered alr fragment was ligated with the expression vector pET24a treated with the same restriction enzyme digestion treatment with T4DNA ligase overnight at 16°C, transformed into E. coli DH5α competent cells by the calcium chloride method, spread on LB plates containing Kan, and cultured overnight at 37°C . Pick a single clone and inoculate it in an LB test tube for culture. Use a plasmid extraction kit to extract the plasmid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com