Pharmaceutical composition of paclitaxel injection
A paclitaxel and composition technology, applied in the field of pharmaceutical compositions containing paclitaxel injections, can solve the problems of less clinical use, etc., and achieve the effects of rapid dissolution and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
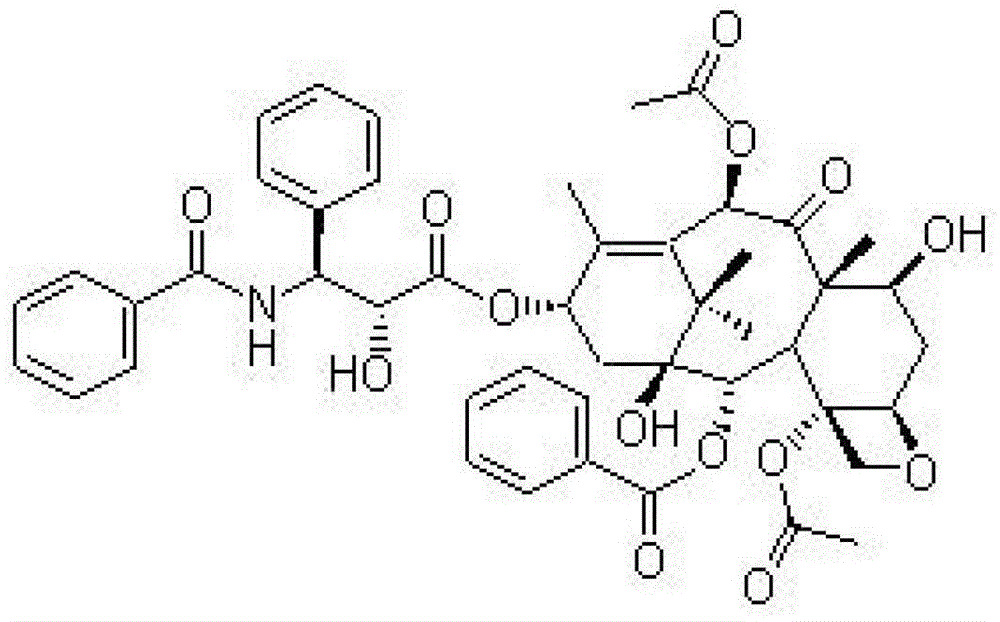
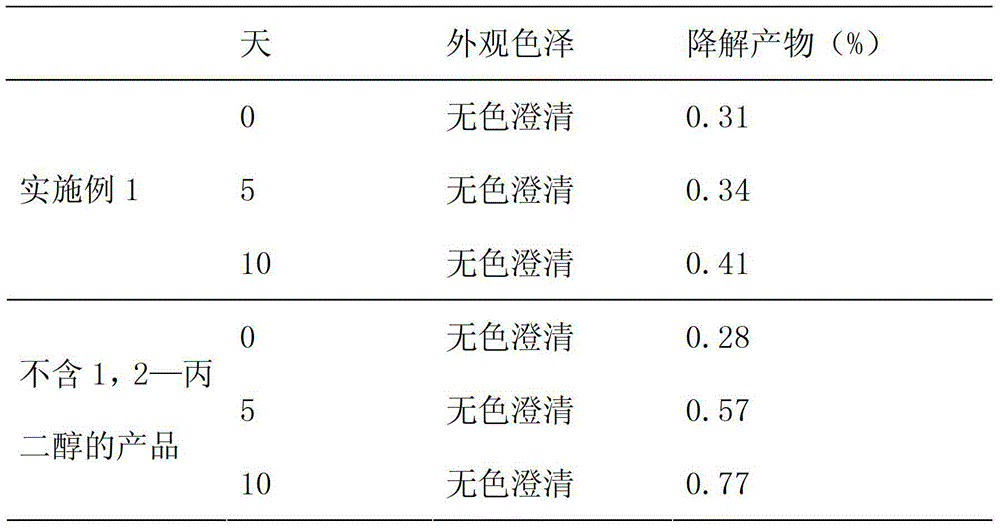
[0045] Weigh 10g of sodium acetylsalicylate, 1g of 1,2-propylene glycol, 2g of paclitaxel plus 900ml of absolute ethanol; add 1g of activated carbon, heat and reflux for 30 minutes, filter and decarbonize, add absolute ethanol to 1000ml, and pass through a 0.45μm micropore Membranes, aseptically filled in vials, 10ml each, sterilized by circulating steam at 110°C for 1 hour.
Embodiment 2
[0047] Weigh 10g of sodium acetylsalicylate, 1g of 1,2-propylene glycol, 2g of paclitaxel plus 900ml of absolute ethanol; add 1g of activated carbon, heat and reflux for 30 minutes, filter and decarbonize, add absolute ethanol to 1000ml, and pass through a 0.45μm micropore Membranes, aseptically filled in medicine bottles, each 20ml, sterilized by circulating steam at 110°C for 1 hour.
Embodiment 3
[0049] Weigh 10g of sodium acetylsalicylate, 1g of 1,2-propylene glycol, 2g of paclitaxel plus 900ml of absolute ethanol; add 1g of activated carbon, heat and reflux for 30 minutes, filter and decarbonize, add absolute ethanol to 1000ml, and pass through a 0.45μm micropore Membranes, aseptically filled in vials, 50ml each, sterilized by circulating steam at 110°C for 1 hour.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com