Phloroglucinol glucoside derivatives and preparation method thereof
A technology of phloroglucinol galactose and lactose, which is applied in the field of sugar engineering, can solve the problems of unstable photolysis and poor water solubility, and achieve the effect of increasing water solubility and stability and expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Phloroglucinol glycoside derivatives, the preparation steps are as follows:
[0028] 1. Preparation of β-galactosidase
[0029] Artificially synthesized β-galactosidase gene sequence with GenBank accession number EU734748.1 (encoded protein GenBank accession number is ACE06986.1), ligated it to pET-21b(+) plasmid, and transformed Escherichia coli BL21(DE3) , and then extract the recombinant plasmid, use the plasmid as a template, and introduce a mutation into the enzyme gene sequence by PCR using a mutation kit (Quanjin Easy Mutagenesis System, Beijing);
[0030] The upstream primer is: 5′-CGGGGATGACTCC TTT GGGCAGAAGGTCCA-3'; SEQ ID NO.3
[0031] The downstream primer is: 5′- AAA GGAGTCATCCCCGCCGACCCCCCATCTG-3'; SEQ ID NO.4
[0032] In the primer, TTT encodes phenylalanine, and tryptophan at position 980 is replaced by phenylalanine by PCR;
[0033] PCR amplification conditions were: pre-denaturation at 95°C for 5 minutes; 20 cycles of reaction (denaturation at 9...
Embodiment 2
[0054] The preparation method of phloroglucinol galactoside, following steps are as follows:
[0055] (1) Use phosphate buffer to prepare a reaction system in which the concentration of lactose is 0.2M, the concentration of phloroglucinol is 0.05M, and the amino acid sequence is as shown in SEQID NO.1, and the amount of β-galactosidase added is 10 μg / mL;
[0056] (2) React the reaction system prepared in step (1) in a water bath at 37°C for 20 minutes, boil to terminate the reaction, centrifuge at 12,000 rpm for 20 minutes, and take the supernatant;
[0057] (3) Purification and detection of phloroglucinol galactoside derivatives are the same as in Example 1.
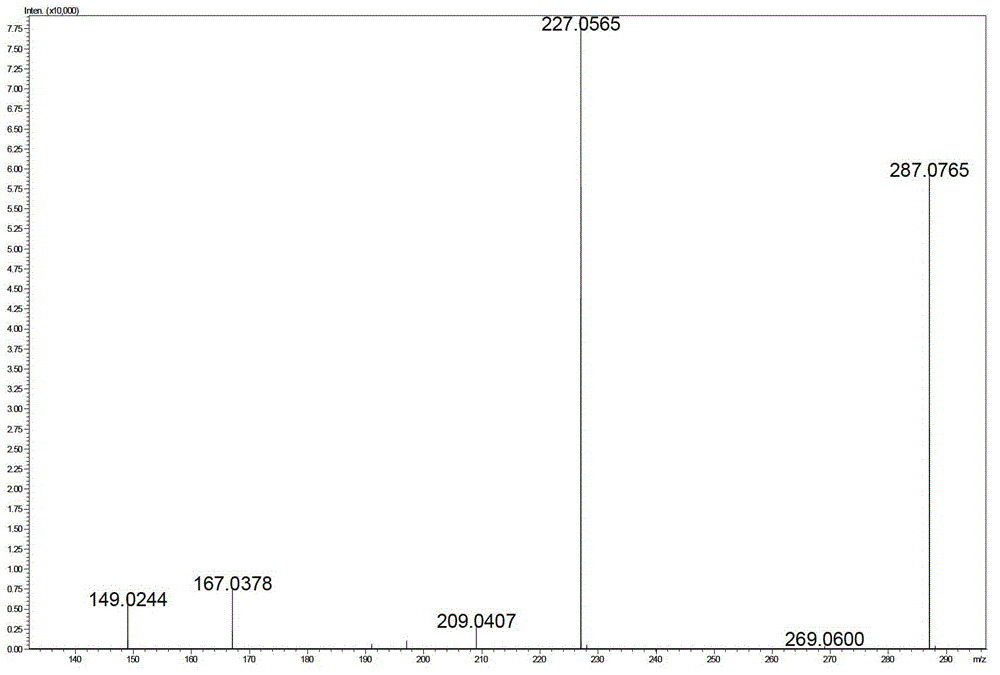
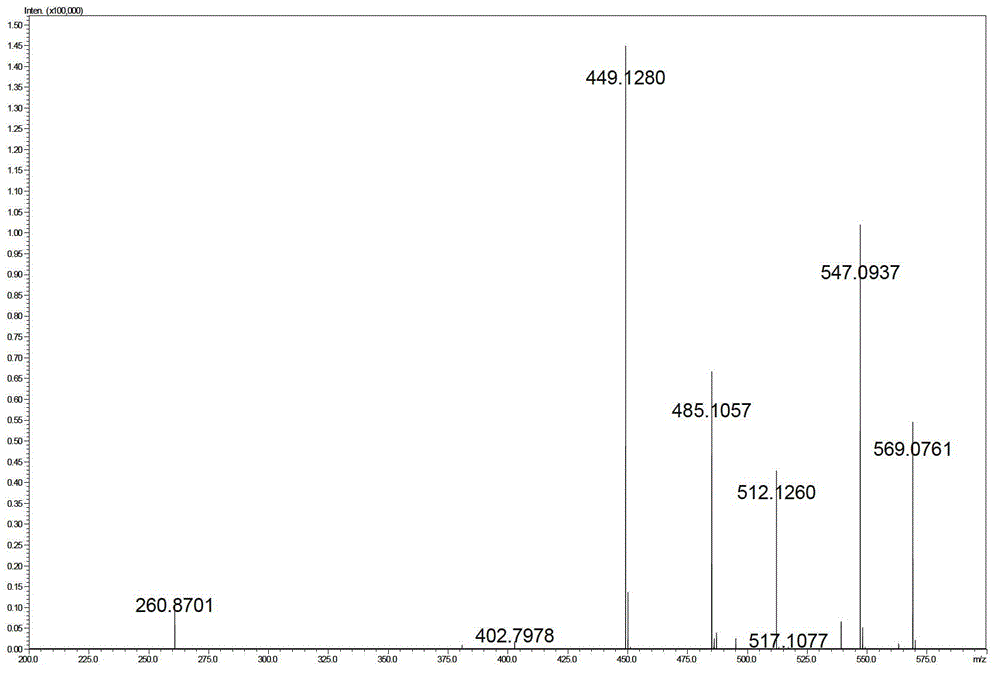
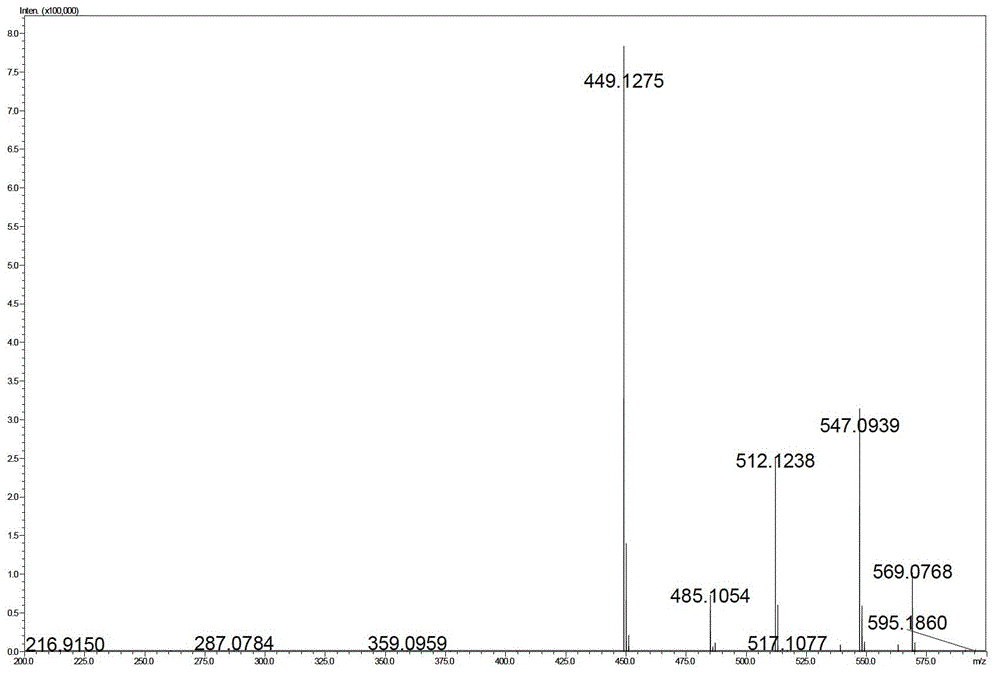
[0058]
[0059]
[0060]
[0061]
[0062]
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com