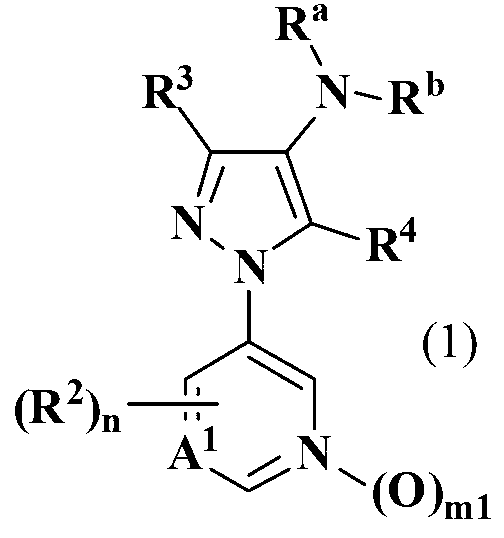
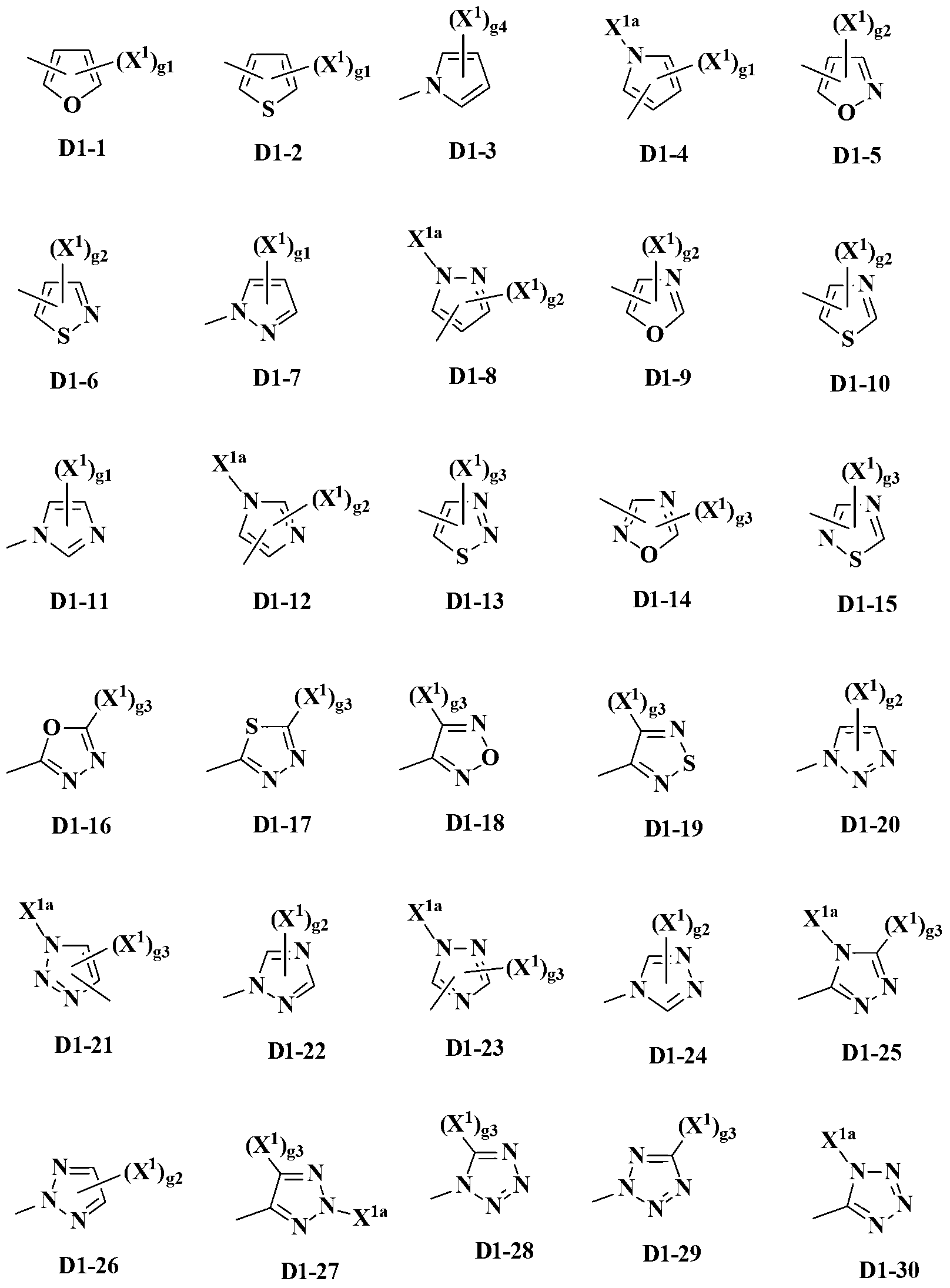
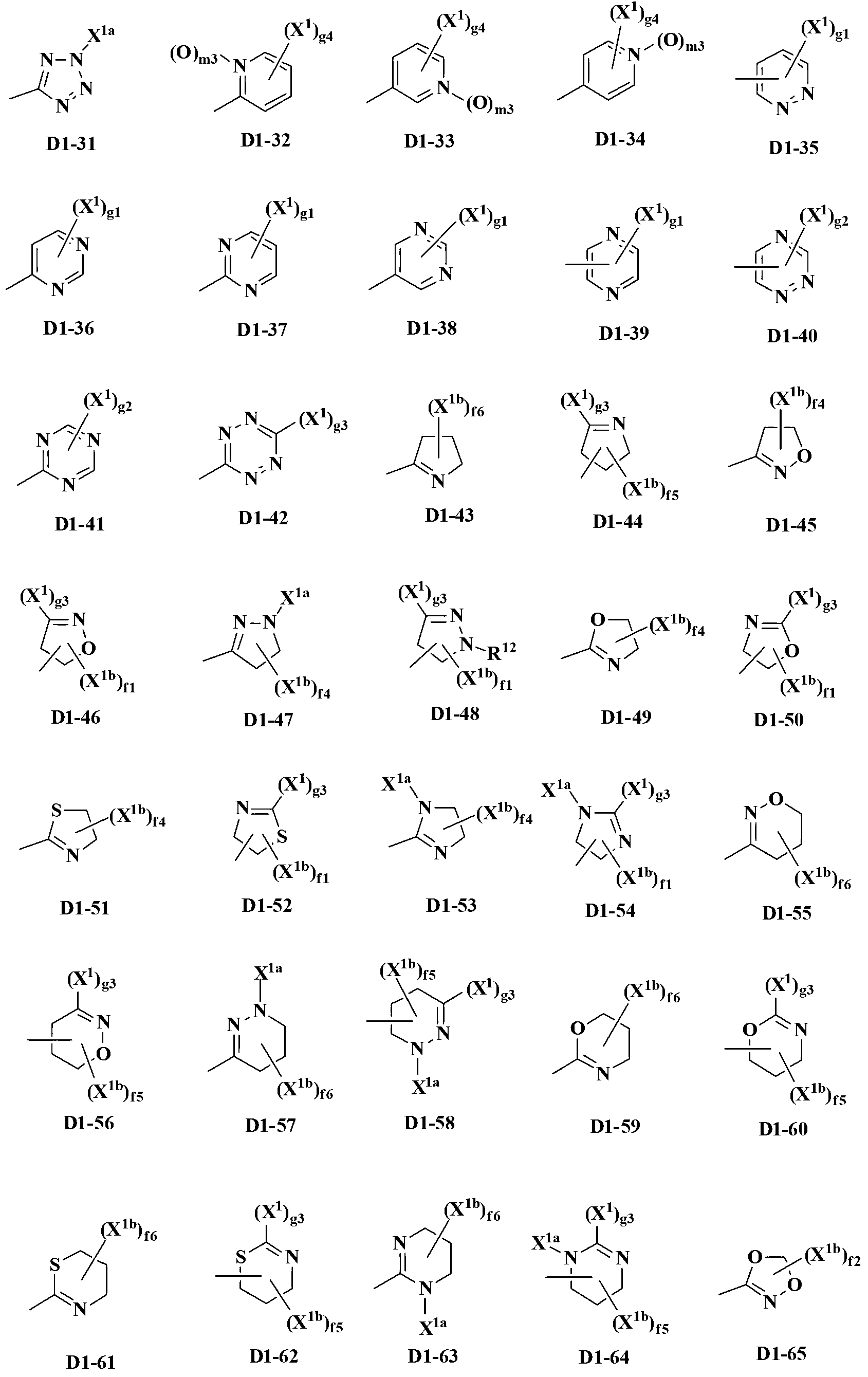
Pyrazole derivative and pest control agent
A kind of pyrazole derivatives, any technology, applied in biocides, animal repellents, plant growth regulators, etc., can solve the problems of undisclosed pyrazole derivatives, unknown usefulness of pest control agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[1129] Hereinafter, the present invention will be further described in detail by specifically describing the synthesis examples and test examples of the compounds of the present invention as examples, but the present invention is not limited thereto.
[1130] The medium pressure fractionation liquid chromatography (Japanese: medium pressure fractionation liquid chromatographic graph) described in the synthesis example used a medium pressure fractionation device manufactured by Yamazen Co., Ltd. (Yazen Co., Ltd.): YFLC-Wprep (flow rate 18ml / min, 40μm silica gel column).
Synthetic example 1
[1133] Production of tert-butyl 3-methyl-1-(pyridin-3-yl)-1H-pyrazol-4-ylcarbamate (Compound 1-011 of the present invention)
[1134] step 1
[1135] Production of ethyl 3-methyl-1-(pyridin-3-yl)-1H-pyrazole-4-carboxylate
[1136] Add 5.3 g of 3-iodopyridine and 990 mg of copper iodide (monovalent) to a solution of 3.0 g of ethyl 3-methyl-1H-pyrazole-4-carboxylate in 10 ml of N,N-dimethylformamide 17.0 g of cesium carbonate. After replacing the inside of the reaction container with nitrogen, it stirred at 120 degreeC for 4 hours. After completion of the reaction, 100 ml of water was added to the reaction mixture, followed by extraction with ethyl acetate (150 ml×1). The obtained organic layer was dehydrated and dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The resulting residue was eluted with n-hexane-ethyl acetate {1:1 (volume ratio, the same applies hereinafter)}, and purified by silica gel column chromatography to obtain...
Synthetic example 2
[1148] Production of tert-butyl methyl{3-methyl-1-(pyridin-3-yl)-1H-pyrazol-4-yl}carbamate (Compound 1-012 of the present invention)
[1149] 3-Methyl-1-(pyridin-3-yl)-1H-pyridine was added to a solution of 83 mg of 60% by weight sodium hydride (dispersed in mineral oil) in 5 ml of N,N-dimethylformamide under ice cooling. tert-butyl azol-4-ylcarbamate 500mg. After the addition was complete, warm to room temperature and stir for 30 minutes. After the stirring was completed, 284 mg of methyl iodide was added to the reaction mixture, and stirring was continued at room temperature for 30 minutes. After the reaction, the reaction mixture was poured into ice water and extracted with 10 ml of ethyl acetate. The obtained organic layer was dehydrated and dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The resulting residue was eluted with n-hexane-ethyl acetate (2:1) and purified by medium pressure fractionation liquid chromatography to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com