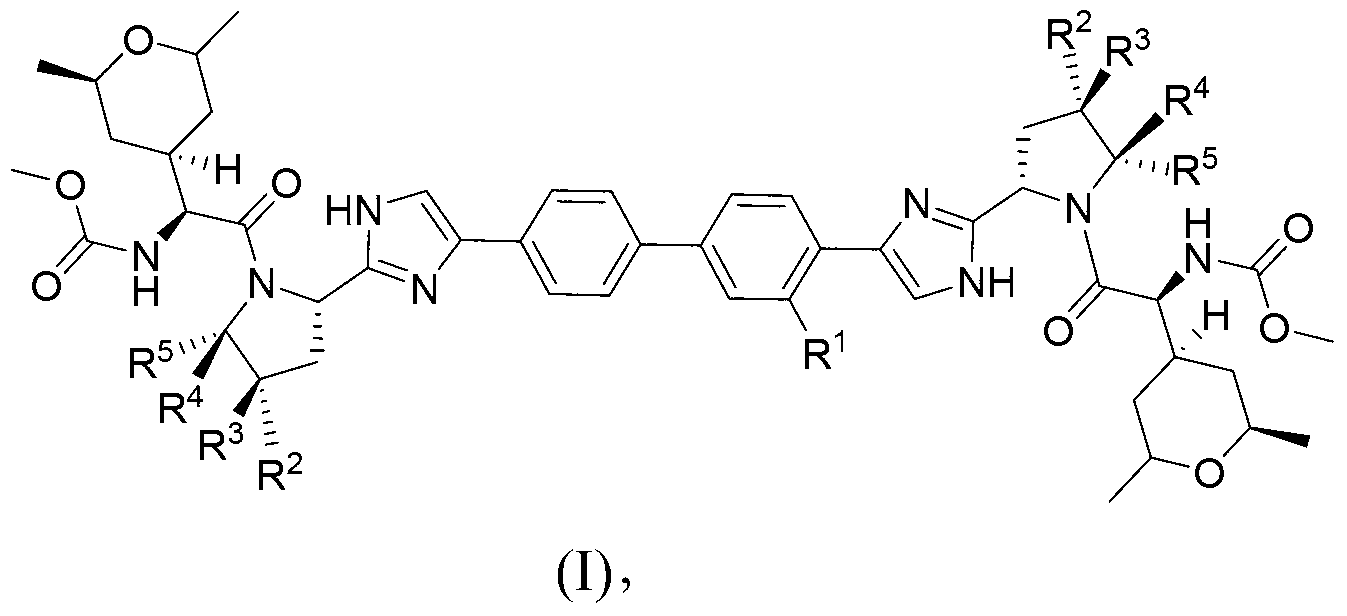
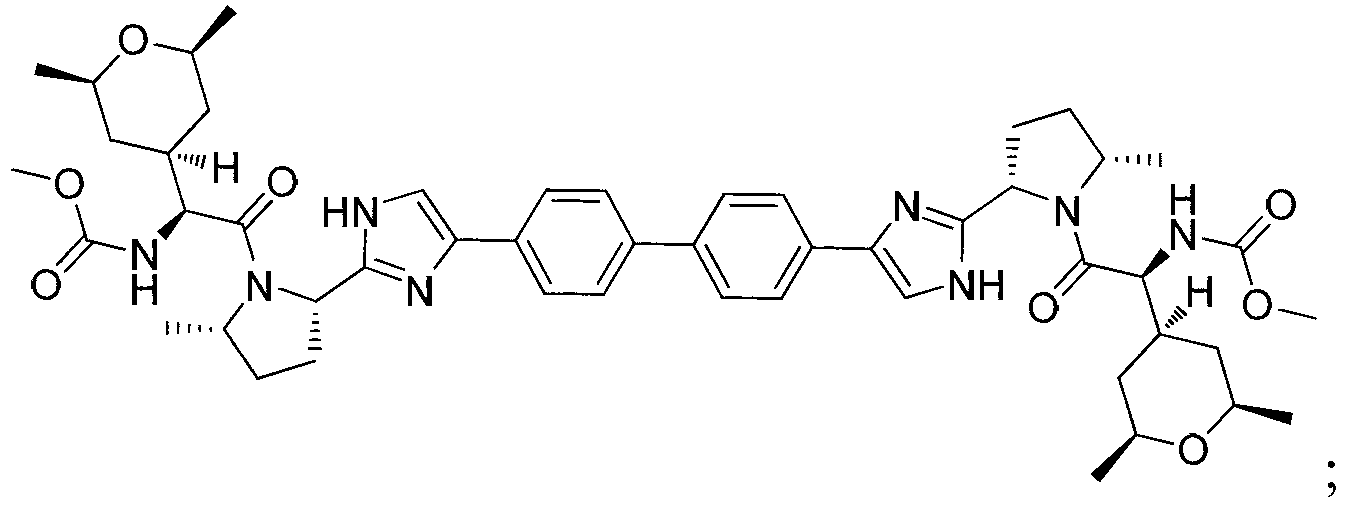
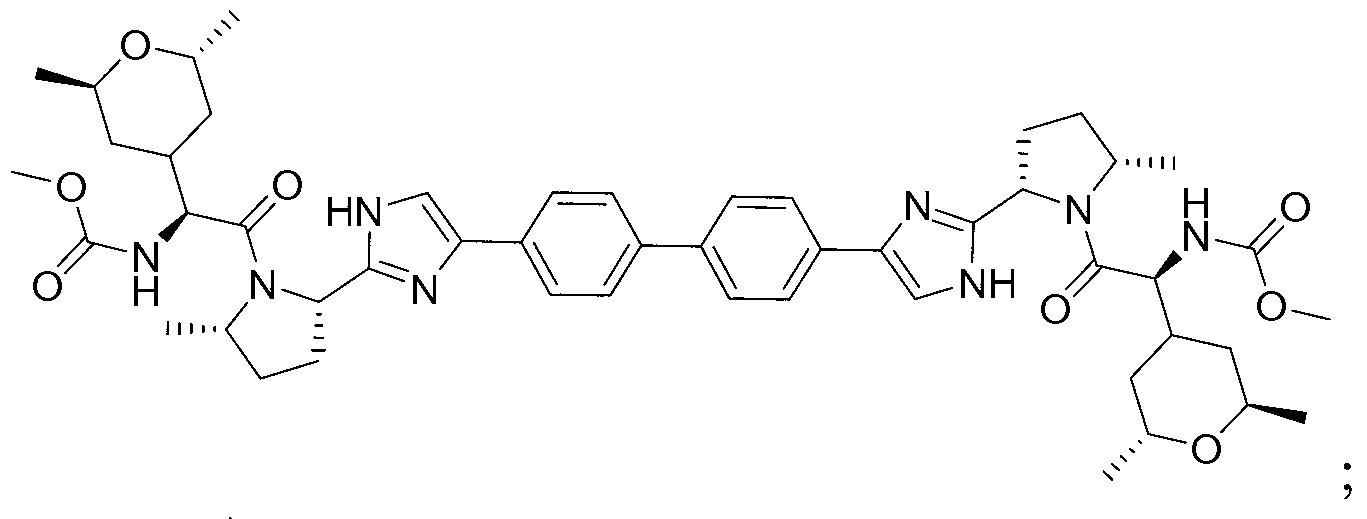
Hepatitis C virus inhibitors
A technology of cyclopropyl rings and compounds, applied in the field of antiviral compounds, can solve the problem of elusive regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186]
[0187] (Biphenyl-4,4'-diylbis(1H-imidazole-4,2-diyl((2S,5S)-5-methyl-2,1-pyrrolidinediyl)((1S)-1 -((2R,6S)-2,6-Dimethyltetrahydro-2H-pyran-4-yl)-2-oxo-2,1-ethanediyl)))dicarbamate dimethyl
[0188] Embodiment 1 step a
[0189]
[0190] The above compound was synthesized according to the literature protocol (J.Med.Chem., 2006, 49, 3520), purified with the following modifications: Recrystallization of the crude material from EtOAc / hexane at ambient temperature afforded Example 1 step as white crystals a. 1 H NMR (400MHz, CDCl 3 )δppm4.32(br m,1H),3.89(br m,1H),2.40(brm,1H),2.00(m,2H),1.65(m,1H),1.45(s,9H),1.20(d ,J=5.6,3H). LC / MS: [M+Na] + C 11 h 20 NO 4 Analytical calculated for Na 252.12; found 252.21.
[0191] Embodiment 1 step b
[0192]
[0193] To Example 1 step a (7.12g, 31.1mmol) and 1,1'-(biphenyl-4,4'-diyl)bis(2-bromoethanone) (6.0g, 15mmol) in acetonitrile (100mL) Add i-Pr dropwise to the mixture in 2 EtN (5.56 mL, 31.8 mmol), and the rea...
Embodiment 2
[0206]
[0207] (Biphenyl-4,4'-diylbis(1H-imidazole-4,2-diyl((2S,5S)-5-methyl-2,1-pyrrolidinediyl)((1S)-1 -((2R,6R)-2,6-Dimethyltetrahydro-2H-pyran-4-yl)-2-oxo-2,1-ethanediyl)))dicarbamate dimethyl
[0208] HATU (63.6 mg, 0.167 mmol) was added to (S)-2-((2R,6R)-2,6-dimethyltetrahydro-2H-pyran-4-yl)-2-(methoxycarbonyl Amino)acetic acid (Cap-2) (41 mg, 0.17 mmol) and the hydrochloride salt (45.5 mg, 0.076 mmol) of step d of Example 1 in DMF (0.9 mL) and DIPEA (0.11 mL, 0.61 mmol) were stirred in solution. The reaction mixture was stirred at room temperature for 4 hours, then concentrated overnight under nitrogen flow. The residue was dissolved in MeOH, filtered and purified by preparative HPLC (Phenomenex Luna C18(2) 100 x 30 mm, 10 microns; MeOH / water) to give (biphenyl-4,4'- Diylbis(1H-imidazole-4,2-diyl((2S,5S)-5-methyl-2,1-pyrrolidinediyl)((1S)-1-((2R,6R)-2 , TFA salt of dimethyl 6-dimethyltetrahydro-2H-pyran-4-yl)-2-oxo-2,1-ethanediyl))) dicarbamate (Example 2) (62.5...
Embodiment 3
[0223]
[0224] ((3-Methyl-biphenyl-4,4'-diyl)bis(1H-imidazol-4,2-diyl((2S,5S)-5-methyl-2,1-pyrrolidinyl )((1S)-1-((2R,6R)-2,6-dimethyltetrahydro-2H-pyran-4-yl)-2-oxo-2,1-ethanediyl))) Dimethyl dicarbamate
[0225] Embodiment 3 step a
[0226]
[0227] To a solution of 4-bromo-2-methylbenzoic acid (10 g, 46.5 mmol) in DMF (150 mL) was added sequentially N,O-dimethylhydroxylamine hydrochloride (5.44 g, 55.8 mmol) at room temperature HOBT (8.55 g, 55.8 mmol). Then EDC (10.7 g, 55.8 mmol), DIPEA (24.4 mL, 140 mmol) were added sequentially and the reaction mixture was stirred at room temperature for 12 hours. The reaction mixture was then diluted with EtOAc (150 mL), washed with water (3 x 250 mL) and brine (150 mL), washed over Na 2 SO 4 Drying, filtration and concentration in vacuo afforded crude Example 3, step a (9.5 g), which was used as such in the next step. 1 H NMR (CDCl 3 ,δ=7.26ppm,400MHz):δ7.37(d,J=1.6Hz,1H),7.34(dd,J=8.0,1.6Hz,1H),7.14(d,J=8.0Hz,1H),3.47 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com