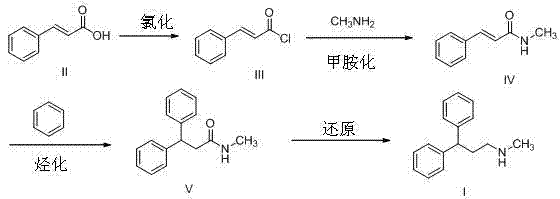
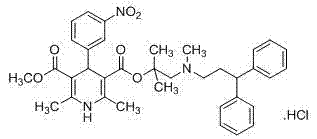
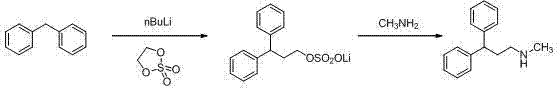
Synthesis method of lercanidipine intermediates
A technology of a dipine intermediate and a synthesis method, which is applied in the field of synthesis of lercanidipine intermediates and can solve the problems of low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of compound III by chlorination of compound II
Embodiment 1-1
[0036] Example 1-1 29.6 g (0.2 mol) of compound II was dissolved in 200 mL of chloroform, and the temperature was raised to 50°C. 14.3 mL (0.2 mol) of thionyl chloride was added dropwise and reacted for 5 h. The reaction liquid was concentrated to obtain a yellow oil (compound III), weighing 33.3 g, which was directly used in the next reaction.
Embodiment 1-2
[0037] Example 1-2 29.6 g (0.2 mol) of Compound II was dissolved in 250 mL of dichloromethane and cooled to 0°C. 99.7 mL (1.4 mol) of thionyl chloride was added dropwise and reacted for 20 h. The reaction solution was concentrated to obtain a yellow oil (compound III), weighing 34.3 g, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com