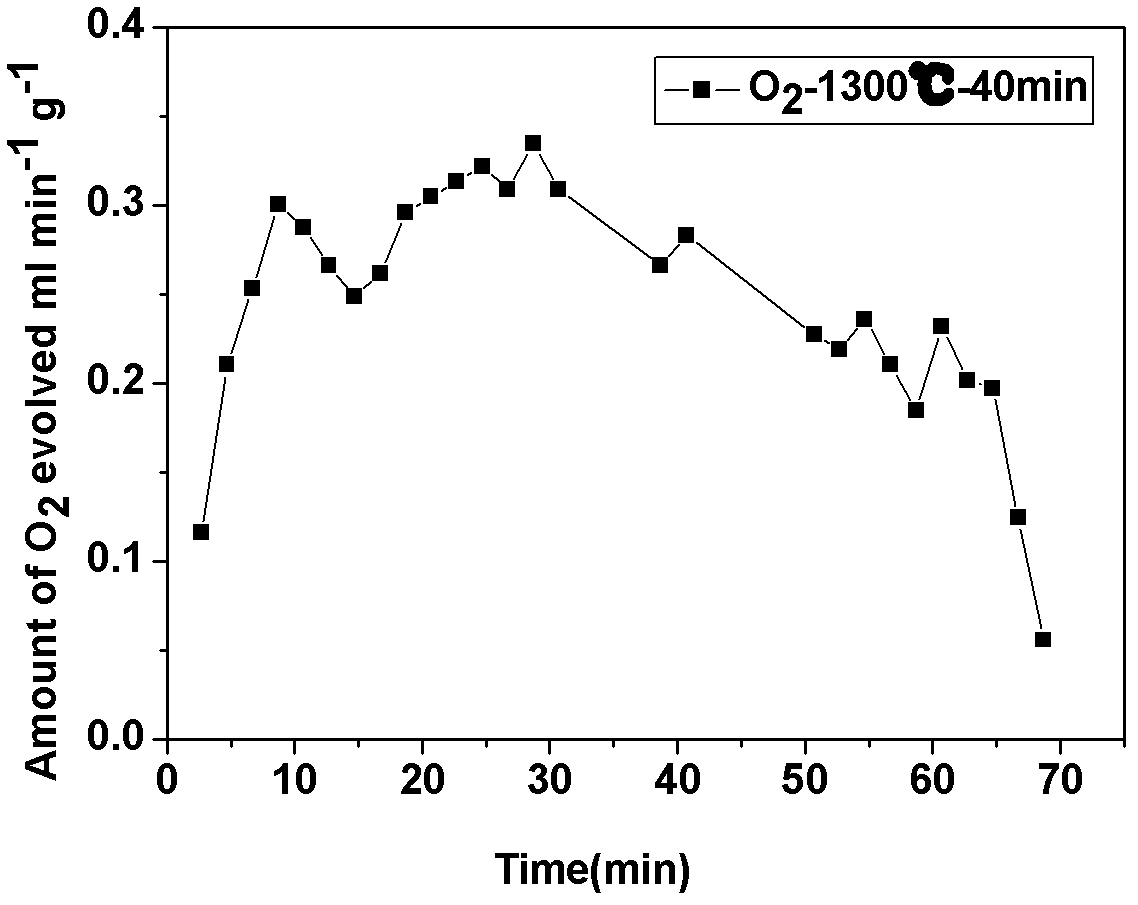
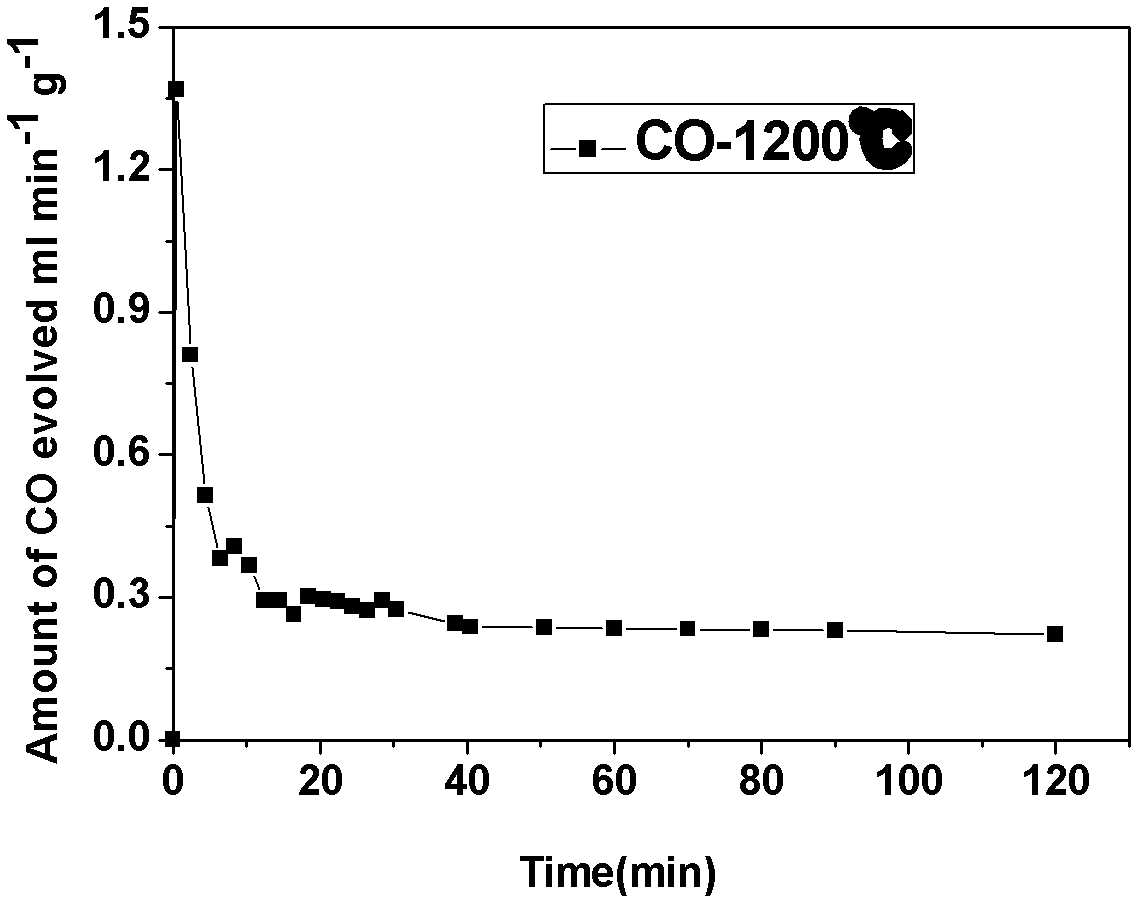
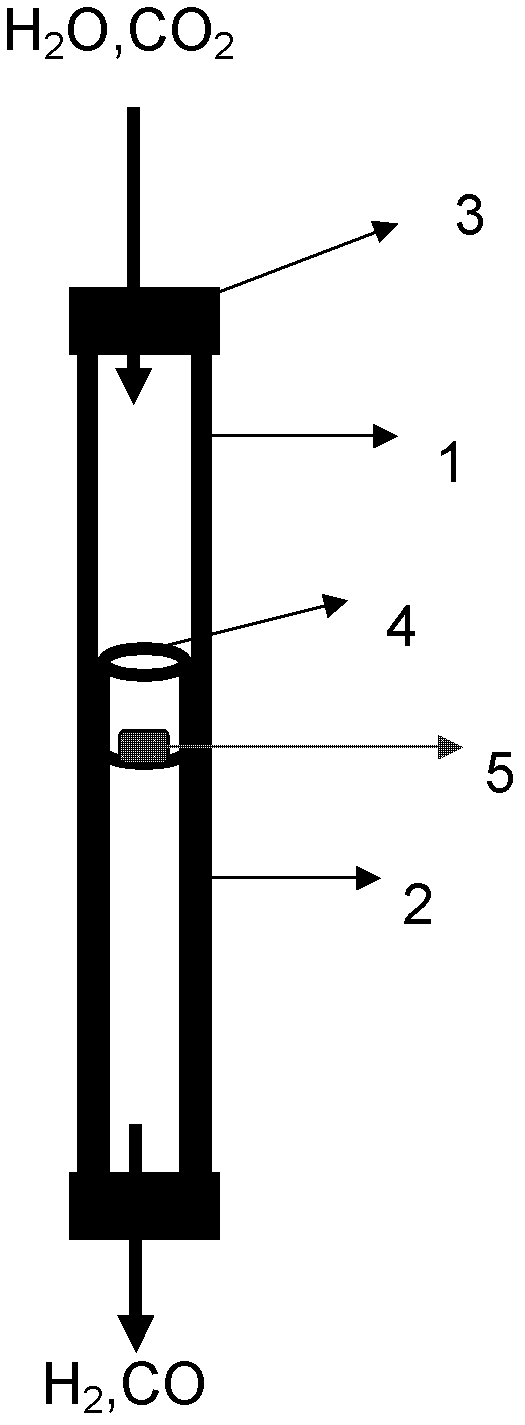
Supported perovskite compound as well as preparation and application thereof
A technology of perovskites and compounds, applied in carbon monoxide, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as poor cycle performance, less oxygen vacancies, and high reduction temperature of metal oxides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] a: Weigh 4.33g of lanthanum nitrate (10mmol), 4.04g of ferric nitrate (10mmol), and 7.6856g of citric acid and dissolve them in 100ml of deionized water, stir at room temperature for 30min, stir in an 80-degree water bath until evaporated, and place in Dried in an oven at 120°C until foaming, milled, and calcined at 700°C for 4 hours, the prepared LaFeO 3 sample.
[0048] b: Mechanical mixed load. Weigh the inert high temperature resistant oxide carrier SiO according to the mass ratio of 3:1 2 , the perovskite material LaFeO 3 , ball milled for 8h, mixed evenly and baked at 900°C for 10h.
Embodiment 2
[0050] a: Weigh 4.33g of lanthanum nitrate (10mmol), 2.5101g of manganese nitrate (10mmol), and 7.6856g of citric acid and dissolve them in 100ml of deionized water, stir at room temperature for 30min, stir in an 80-degree water bath until evaporated, and place in Dried in an oven at 120°C until foaming, milled, and calcined at 700°C for 4h, the prepared LaMnO 3 sample.
[0051] b: Mechanical mixed load. Weigh the inert high temperature resistant oxide carrier SiO according to the mass ratio of 3:1 2 , the perovskite material LaMnO 3 , ball milled for 8h, mixed evenly and baked at 900°C for 10h.
Embodiment 3
[0053] a: Weigh 4.33g of lanthanum nitrate (10mmol), 2.9105g of cobalt nitrate (10mmol), and 7.6856g of citric acid and dissolve them in 100ml of deionized water, stir at room temperature for 30min, stir in a water bath at 80°C until evaporated, and place in LaCoO was dried in an oven at 120°C until foaming, milled, and fired at 700°C for 4 hours. 3 sample.
[0054] b: Mechanical mixed load. Weigh the inert high temperature resistant oxide carrier SiO according to the mass ratio of 3:1 2 , the perovskite material LaCoO 3 , ball milled for 8h, mixed evenly and baked at 900°C for 10h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com