The manufacture method of fluoroalkyl iodide
A technology for fluoroalkyl iodides and manufacturing methods, which is applied in the fields of chemical instruments and methods, organic chemistry, preparation of halogenated hydrocarbons, etc., and can solve the problems of increasing reactors, reducing reaction speed, and reducing the selectivity of fluoroalkyl iodides, etc. problems, to achieve the effects of shortened residence time, increased reaction speed, and excellent reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
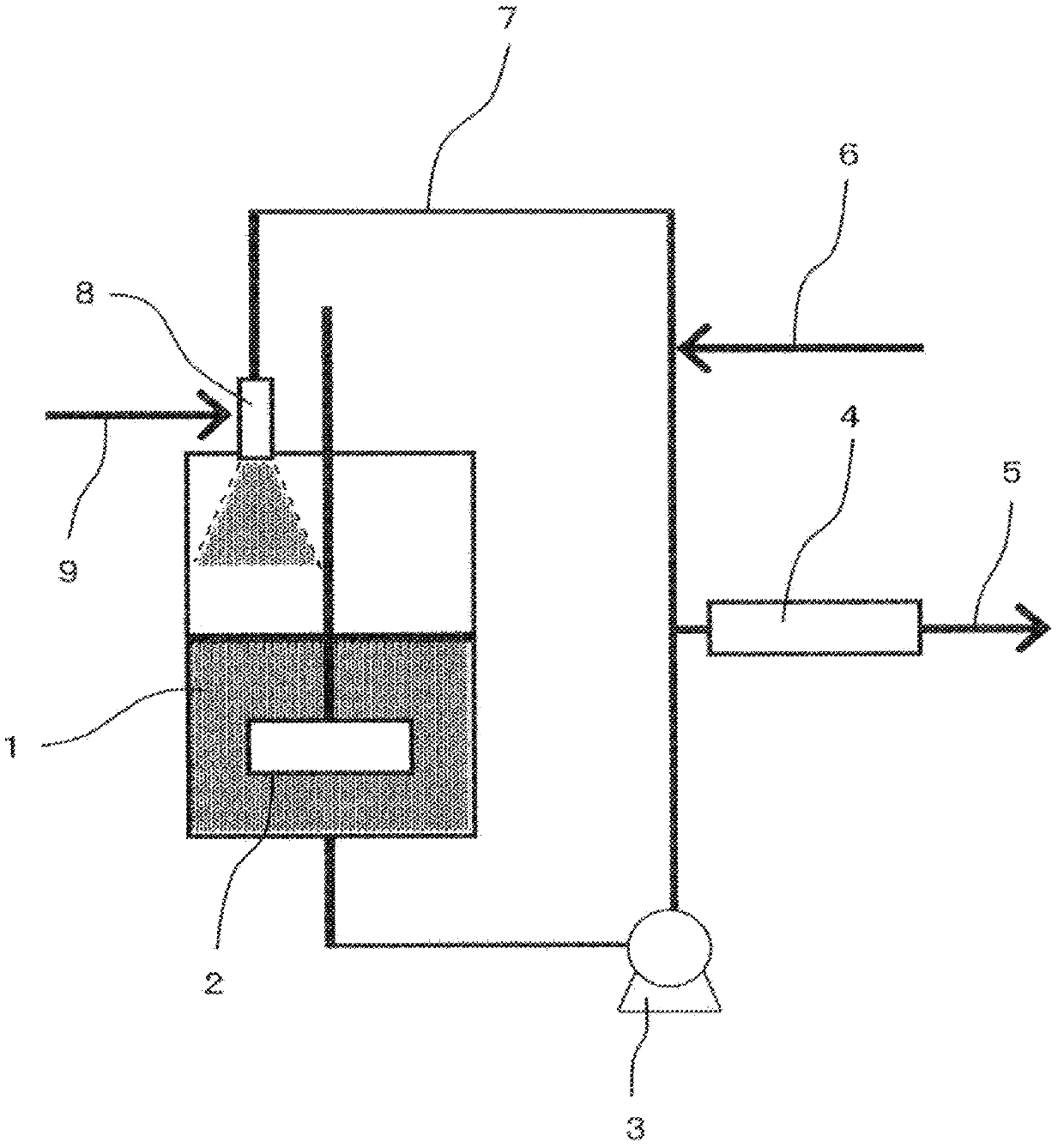
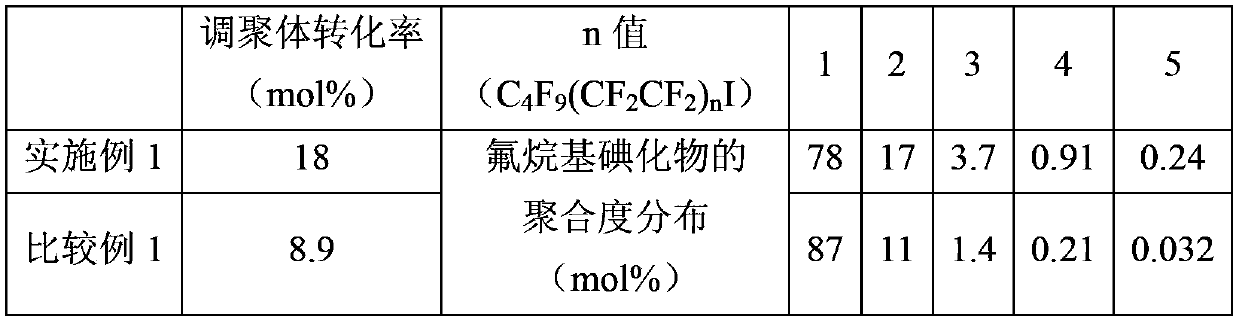
[0075] use as figure 1 The reaction apparatus shown is for telomerization reaction. In a stainless steel pressurized reactor 1 with a capacity of 3 L with a stirrer 2, 1-iodoperfluorobutane (C 4 f 9I) 3.0 kg and 150 g of copper powder as a metal catalyst were stirred by the stirrer 2 . It was circulated by the circulation pump 3, and it heated to 100 degreeC. While maintaining the temperature in the reactor at 100° C., tetrafluoroethylene as the main chain product was supplied to the injector 8 through the piping for feeding the raw material main chain product. Stirring the reaction liquid, the pressure was increased until the pressure in the reactor reached 0.38 MPa (gauge pressure).
[0076] Afterwards, keep above-mentioned temperature and pressure condition, feed 1-iodoperfluorobutane (C 4 f 9 I) Copper powder as a metal catalyst was supplied to the reaction liquid circulation pipe 7 at a flow rate of 150 g / hr. And, supply tetrafluoroethylene to injector 8 with the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com