Roflumilast N-oxide polymorphs and their preparation methods and pharmaceutical composition
A technology of roflumilast and crystal forms, which is applied in the direction of drug combination, active ingredients of heterocyclic compounds, digestive system, etc., and can solve problems such as no description of crystal forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
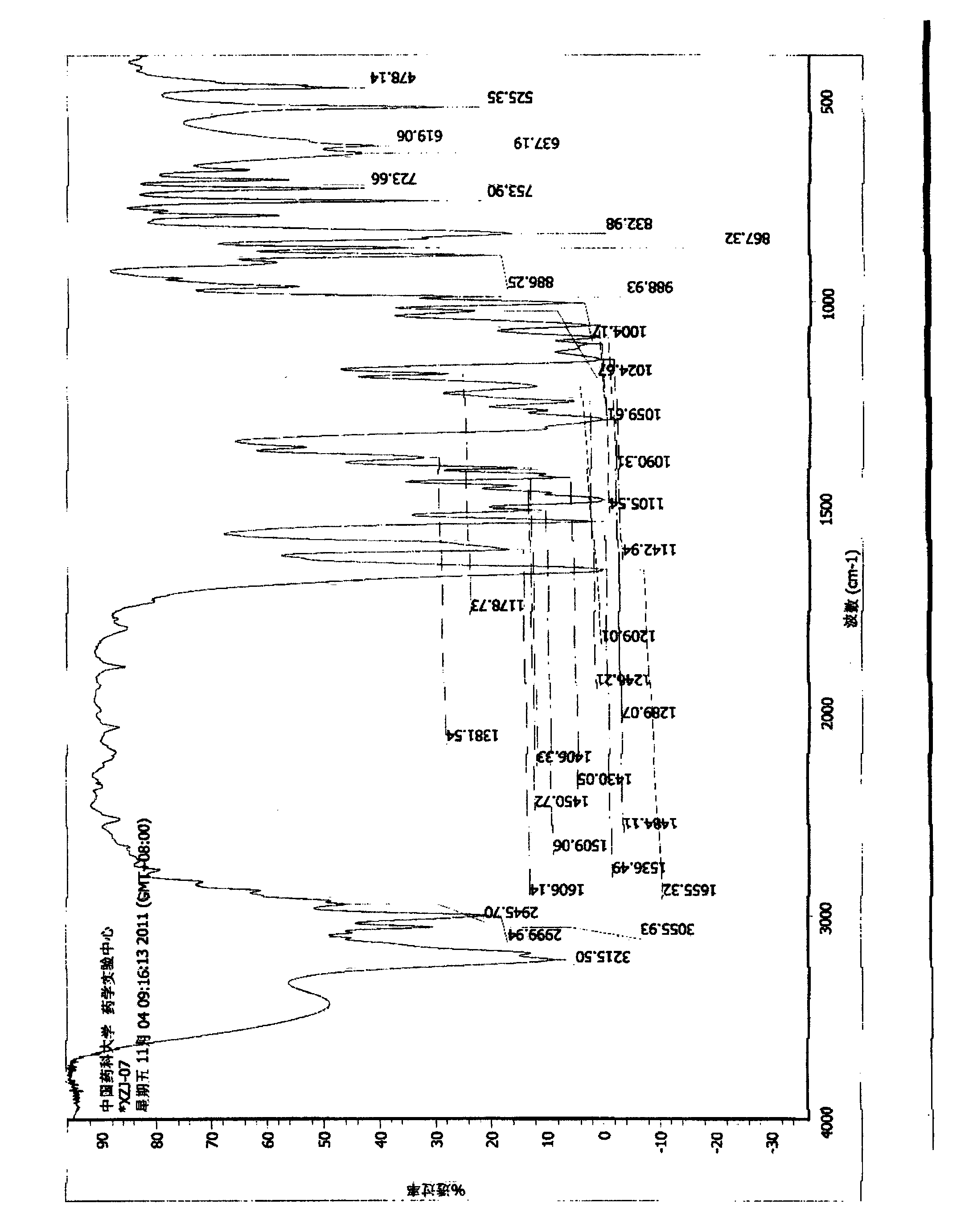
[0057] Form I of roflumilast N-oxide
[0058] Under stirring at a bath temperature of 30° C., 3.1 g of N-(3,5-dichloro-1-oxo-pyridin-4-yl)-3-cyclopropanemethoxy-4-difluoromethoxyformamide ( Roflumilast N-oxide) solid was dissolved in a mixed solvent of dichloromethane-methanol (80:1, 50mL), and the solvent was spin-dried under reduced pressure at 40°C to precipitate a white solid. The obtained solid was collected and dried under reduced pressure at 55°C to obtain 3.1g of a white solid with a melting point of 179°C to 180°C, which was confirmed to be roflumilast N by X-ray powder diffraction, infrared scanning, DSC scanning, TGA scanning, and elemental analysis. - Form I of the oxide.
Embodiment 2
[0060] Form I of roflumilast N-oxide
[0061] Under stirring at a bath temperature of 85°C, dissolve 500 mg of N-(3,5-dichloro-1-oxo-pyridin-4-yl)-3-cyclopropanemethoxy-4-difluoromethoxyformamide as a solid After cooling to 20°C in ethyl acetate (5 mL) and standing still, a white solid precipitated out of the solution. Suction filtration, the filter cake was collected, and the obtained solid was dried at 40°C under reduced pressure to obtain 290 mg of white solid, melting point: 179°C-180°C, which was confirmed to be the crystal form of roflumilast N-oxide by X-ray powder diffraction Object I.
Embodiment 3
[0063] Form I of roflumilast N-oxide
[0064] Under stirring at a bath temperature of 85°C, dissolve 1 g of N-(3,5-dichloro-1-oxo-pyridin-4-yl)-3-cyclopropanemethoxy-4-difluoromethoxyformamide as a solid After cooling to 20°C in acetonitrile (9 mL) and standing still, a white solid precipitated out of the solution. Suction filtration, the filter cake was collected, and the obtained solid was dried at 40°C under reduced pressure to obtain 680 mg of white solid, melting point: 179°C-180°C, which was confirmed to be the crystal form of roflumilast N-oxide by X-ray powder diffraction Object I.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com