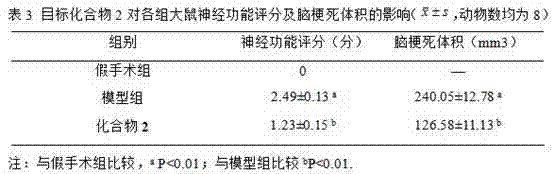
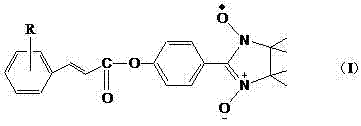
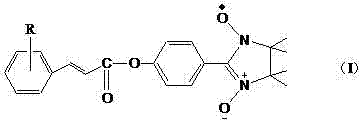
Application of free radical of nitroxide to treatment of ischemia reperfusion injury
A technology of ischemia-reperfusion and selection, which is applied in the field of medicine and can solve the problems of large clinical gaps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the synthetic method of compound 1
[0017]
[0018] (1) Synthesis of 4-hydroxyphenylacrylic acid chloride
[0019] Dissolve 1.64 g (10.0 mmol) p-hydroxyphenylacrylic acid in 50 mL CH 2 Cl 2 , cooled in an ice-water bath, and added dropwise 10.0 mL SOCl 2 (1.18 g) CH 2 Cl 2 solution, and then warmed to reflux for 2h to stop the reaction. The solvent was removed under reduced pressure and used directly in the next reaction.
[0020] (2) Synthesis of p-hydroxyl nitroxide free radicals
[0021] 1.22 g (10.0 mmol) of p-hydroxybenzaldehyde and 1.48 g (10.0 mmol) of dihydroxylamine were dissolved in 50 mL of methanol and refluxed for 24 h. A large amount of white insoluble matter was formed, filtered, and the filter cake was washed with a small amount of methanol. Suspend the filter cake in 50.0 mL CH 2 Cl 2 , cooled in an ice-water bath, and added 30.0 mL NaIO 4 (1.7 g) aqueous solution, the reaction was stopped after stirring for 15 min. Af...
Embodiment 2
[0024] Embodiment 2: the synthetic method of compound 2
[0025]
[0026] (1) Synthesis of 3-methoxy-4-hydroxyphenylacrylic acid chloride
[0027] Dissolve 1.94 g (10.0 mmol) p-hydroxyphenylacrylic acid in 50 mL CH 2 Cl 2 , cooled in an ice-water bath, and added dropwise 10.0 mL SOCl 2 (1.18 g) CH 2 Cl 2 solution, and then warmed to reflux for 2h to stop the reaction. The solvent was removed under reduced pressure for the next reaction.
[0028] (2) compound 2 Synthesis
[0029] Dissolve 2.49 g (10.0 mmol) of p-hydroxyl nitroxide in 50 mL CH 2 Cl 2 , cooled in an ice-water bath, and added dropwise 2.12 g (10.0 mmol) of 3-methoxy-4-hydroxyphenylacrylic acid chloride in 20.0 mL of CH 2 Cl 2 solution, and then warmed to reflux for 2h to stop the reaction. The solvent was removed under reduced pressure, and the product was purified by column chromatography to obtain 3.91 g of the product, with a yield of 92%. mp: 245~247℃. IR ( ν max / cm -1 ): 3485, 1720, 16...
Embodiment 3
[0030] Embodiment 3: the synthetic method of compound 3
[0031]
[0032] (1) Synthesis of 3-hydroxy-4-hydroxybenzeneacrylic acid chloride
[0033] Dissolve 1.64 g (10.0 mmol) p-hydroxyphenylacrylic acid in 50 mL CH 2 Cl 2 , cooled in an ice-water bath, and added dropwise 10.0 mL SOCl 2 (1.18 g) CH 2 Cl 2 solution, and then warmed to reflux for 2h to stop the reaction. The solvent was removed under reduced pressure for the next reaction.
[0034] (2) compound 3 Synthesis
[0035] Dissolve 2.49 g (10.0 mmol) of p-hydroxyl nitroxide in 50 mL CH 2 Cl 2 , cooled in an ice-water bath, and added dropwise 1.98 g (10.0 mmol) of p-hydroxybenzoic acid chloride in 20.0 mL of CH 2 Cl 2 solution, and then warmed to reflux for 2h to stop the reaction. The solvent was removed under reduced pressure, and the product was purified by column chromatography to obtain 3.91 g of the product, with a yield of 95%. mp: 203~204℃. IR ( ν max / cm -1 ): 3480, 1710, 1647, 1509, 1635,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com