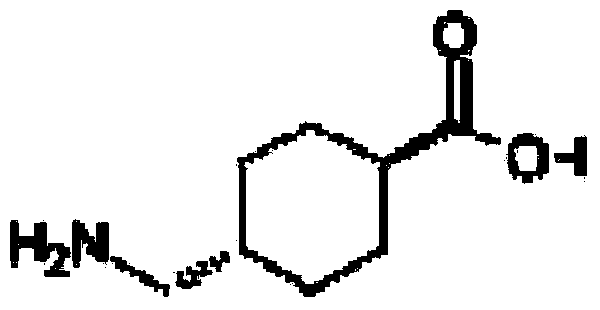
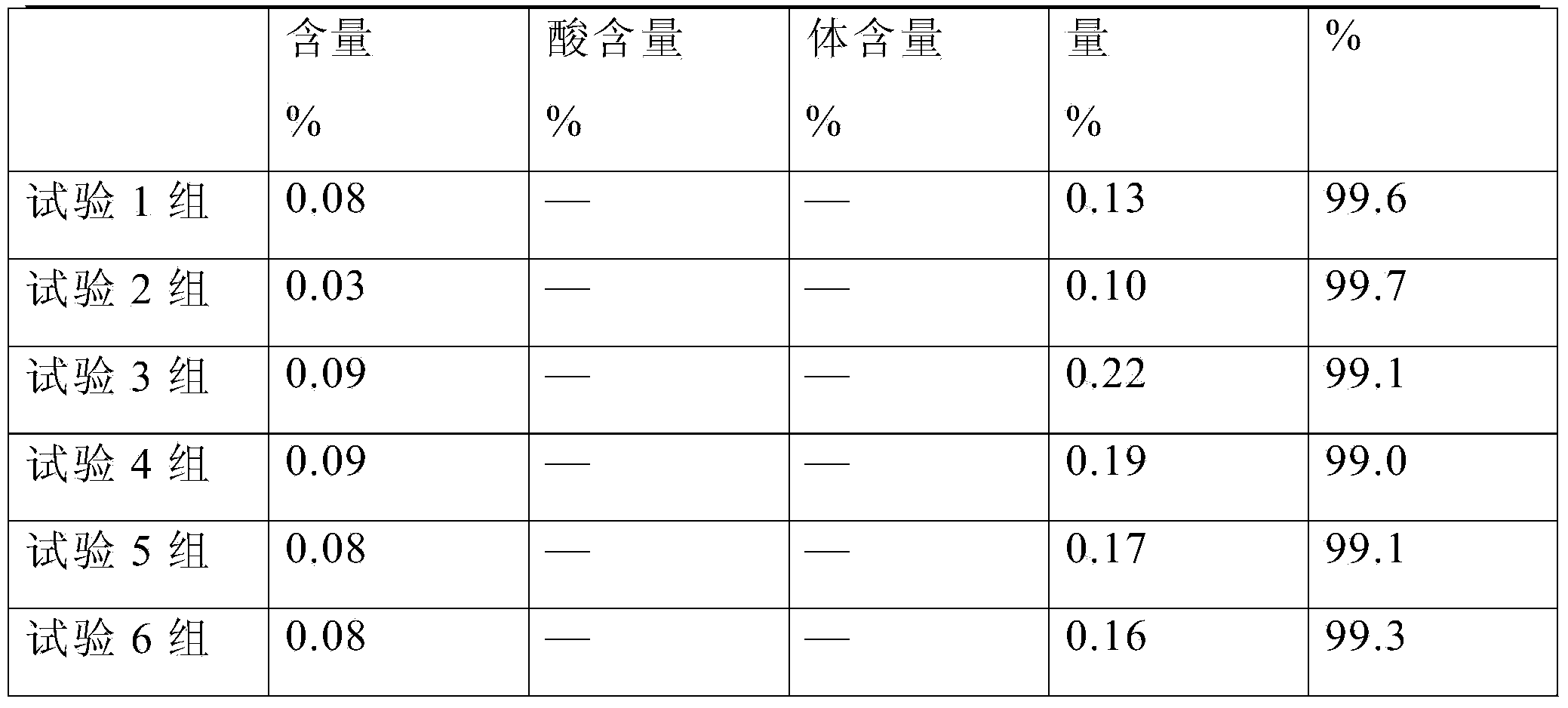
Pharmaceutical composition of tranexamic acid
A technology of tranexamic acid and its composition, which is applied in the field of medicine and can solve the problems of low medical quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Tranexamic acid 200g, citric acid 13.6g, L-glutamic acid 1.36g, water for injection 2000mL.
[0107]Preparation method: Take 1500mL of water for injection, heat to 60°C, add 5g of citric acid, dissolve completely, add tranexamic acid, dissolve completely, add the remaining organic acid, add moistened 0.1% (g / ml) activated carbon for needles , stir evenly, heat-preserve and absorb for 30 minutes, decarbonize and filter through a 0.45 μm microporous membrane while it is hot, and filter it to a dilute tank, take a small amount of water for injection to wash the carbon. Add water for injection to nearly the prescribed amount. Fine filter with 0.22μm microporous membrane, fill with nitrogen and seal in ampoules sterilized at ≥300℃ for ≥5 minutes. Sterilize at 115°C for 30 minutes, pack and obtain 1000 injections.
Embodiment 2
[0109] Tranexamic acid 200g, citric acid 11.5g, L-glutamic acid 3.5g, water for injection 2000mL.
[0110] Preparation method: Take 1500mL of water for injection, heat to 60°C, add 5g of citric acid, dissolve completely, add tranexamic acid, dissolve completely, add the remaining organic acid, add moistened 0.1% (g / ml) activated carbon for needles , stir evenly, heat-preserve and absorb for 30 minutes, decarbonize and filter through a 0.45 μm microporous membrane while it is hot, and filter it to a dilute tank, take a small amount of water for injection to wash the carbon. Add water for injection to nearly the prescribed amount. Fine filter with 0.22μm microporous membrane, fill with nitrogen and seal in ampoules sterilized at ≥300℃ for ≥5 minutes. Sterilize at 115°C for 30 minutes, pack and obtain 1000 injections.
Embodiment 3
[0112] Tranexamic acid 250g, citric acid 14.4g, L-glutamic acid 4.4g, water for injection 5000mL.
[0113] Preparation method: Take 1875mL of water for injection, heat to 60°C, add 6.25g of citric acid, dissolve completely, add tranexamic acid, dissolve completely, add the remaining organic acid, add moistened 0.1% (g / ml) for needle use Activated carbon, stir evenly, heat-preserve and adsorb for 30 minutes, decarbonize and filter through a 0.45 μm microporous membrane while it is hot, and filter it to a dilute tank, take a small amount of water for injection to wash the carbon. Add water for injection to nearly the prescribed amount. Fine filter with 0.22μm microporous membrane, fill with nitrogen and seal in ampoules sterilized at ≥300℃ for ≥5 minutes. Sterilize at 115°C for 30 minutes, pack and obtain 1000 injections.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com