Separation process of substituted benzaldehyde co-produced products
A production process and technology of deionized water, applied in the separation/purification of carboxylic acid compounds, separation/purification of carbonyl compounds, organic chemistry, etc. The effect of wastewater
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
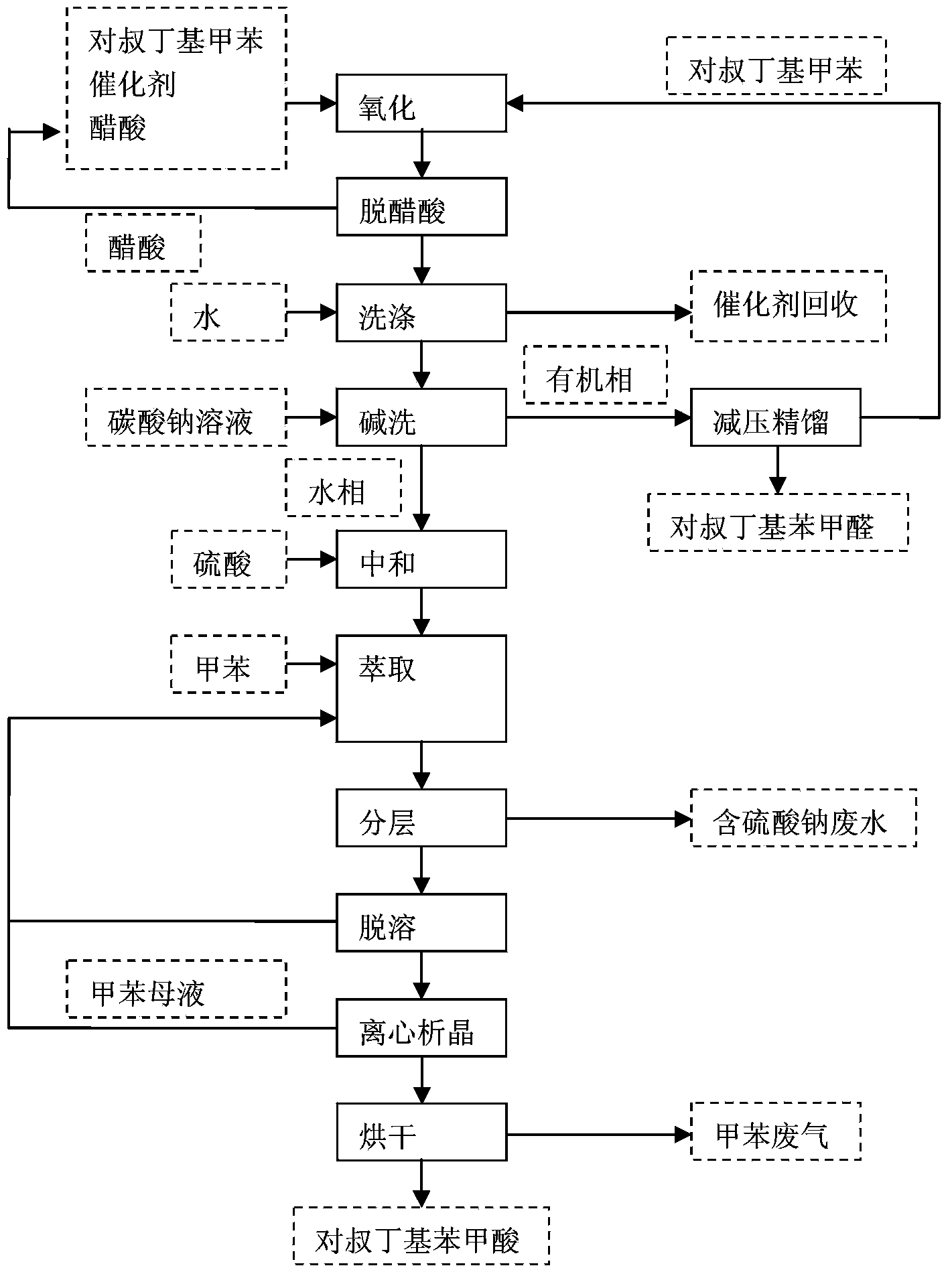
[0014] In a 1000mL flask, add 675mL of p-tert-butyltoluene oxidation feed solution; rectify to remove acetic acid, add water twice to wash off the catalyst in total of 300mL.
[0015] The organic phase after water washing is transferred to a 1000mL three-neck flask with stirring and heating mantle heating, heated to 95°C, and 0.2MPa saturated water vapor is introduced for stripping, and the mixed steam after stripping is sent to the middle end of the fractionation column, and the fractionation column There is a fractionation column bottom bottle at the bottom, and the mixed steam is partially condensed in the fractionation column, and the condensate contacts the steam that continues to rise on the way down, and the two perform heat exchange, and the p-tert-butylbenzaldehyde and p-tert-butyltoluene with high boiling points in the vapor are absorbed Condensation, the water vapor with low boiling point still rises as steam, while the low boiling point components in the condensate ...
Embodiment 2
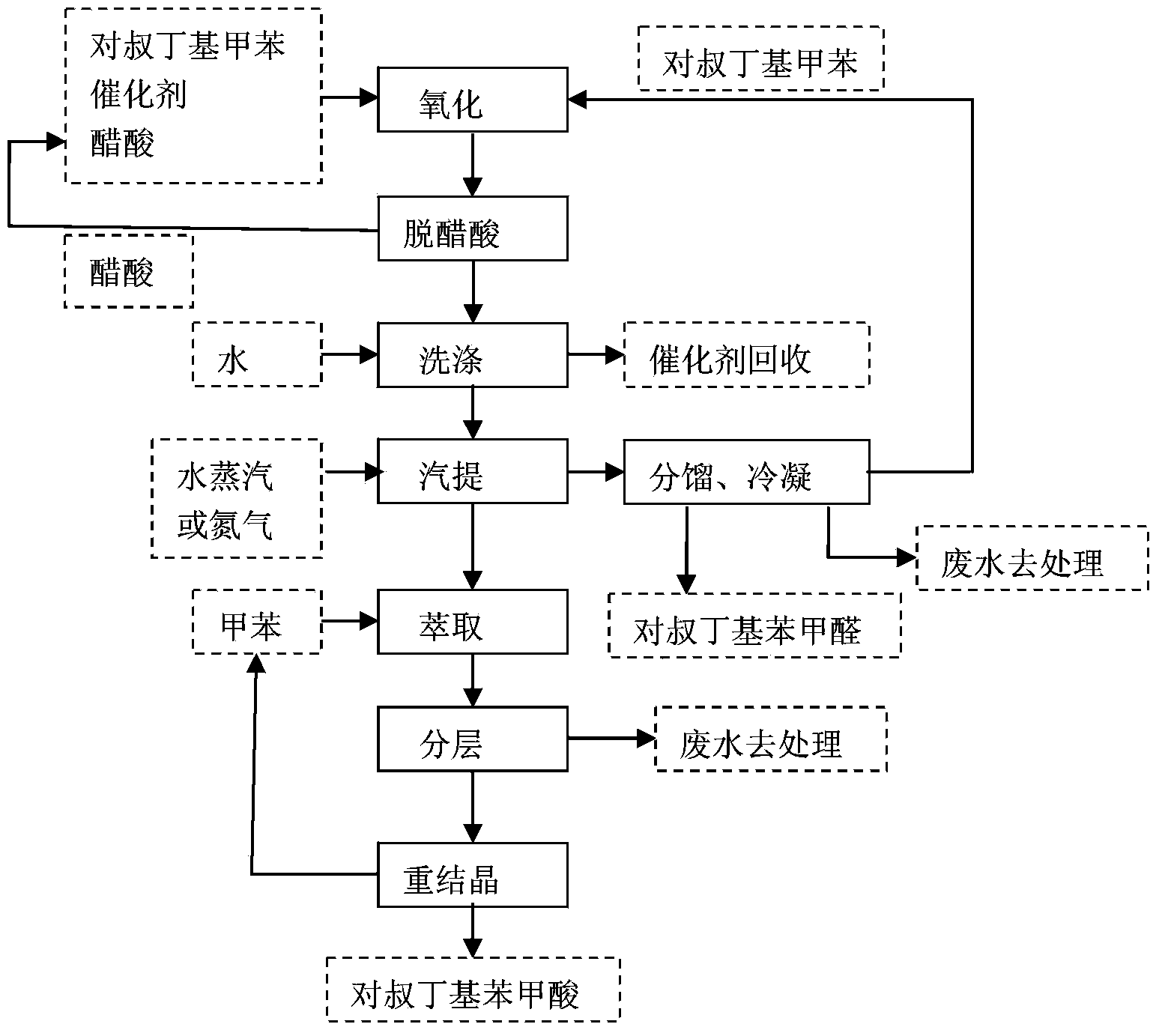
[0019] In a 1000mL flask, add 675mL of p-tert-butyltoluene oxidation feed solution; rectify to remove acetic acid, add water twice to wash off the catalyst in total of 300mL.
[0020] The organic phase after water washing was transferred to a 1000mL three-necked flask with stirring and heating mantle heating, heated to 95°C, and 0.2MPa nitrogen gas was introduced for stripping, and the mixed steam after stripping was sent to the middle end of the fractionation column. In the bottom bottle of the fractionating column, the mixed vapor is partially condensed in the fractionating column, and the condensate contacts the steam that continues to rise during the descent, and the two perform heat exchange, and p-tert-butylbenzaldehyde and p-tert-butyltoluene with high boiling points in the vapor are condensed, The nitrogen with low boiling point still rises as a vapor, while the low boiling point components in the condensate are heated and vaporized, and the high boiling point component...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com