Pridinol methanesulfonate matrix sustained-release tablet and preparation method thereof
A technology of pridinol and methanesulfonic acid, which is applied in bone diseases, medical formulas, medical preparations with non-active ingredients, etc., can solve the problems that there are no related reports on sustained-release tablets, and achieve easy control of the quality of the preparations and the production process. Simple, Prescription Advanced Results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, prescription and process screening of pridinol mesylate matrix sustained-release tablet
[0027] 1. Single factor test
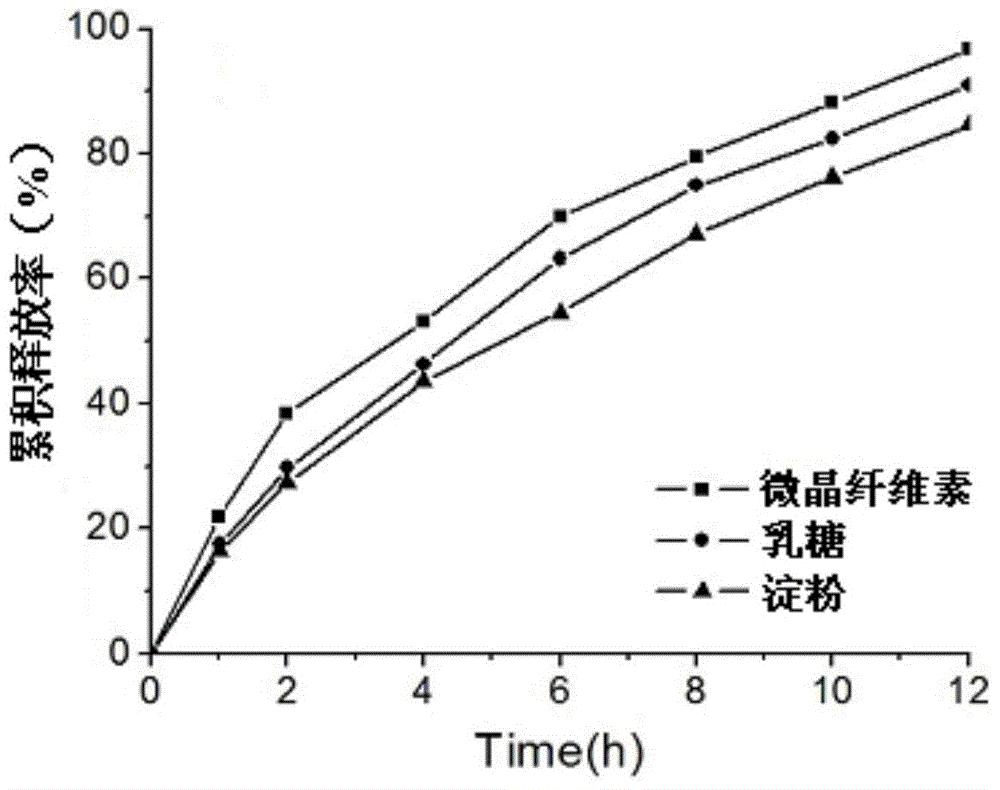
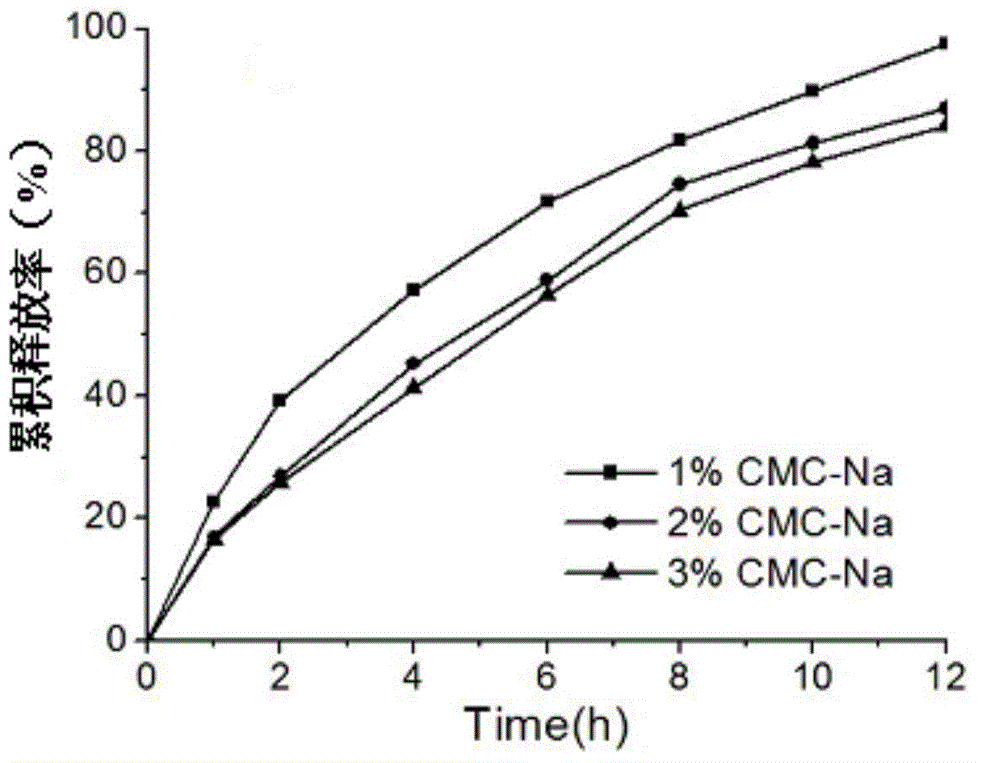
[0028] Taking the in vitro release rate as the index, the influence of matrix materials, fillers, binders, blockers and tableting pressure on the release rate of pridinol mesylate matrix sustained-release tablets was mainly investigated.
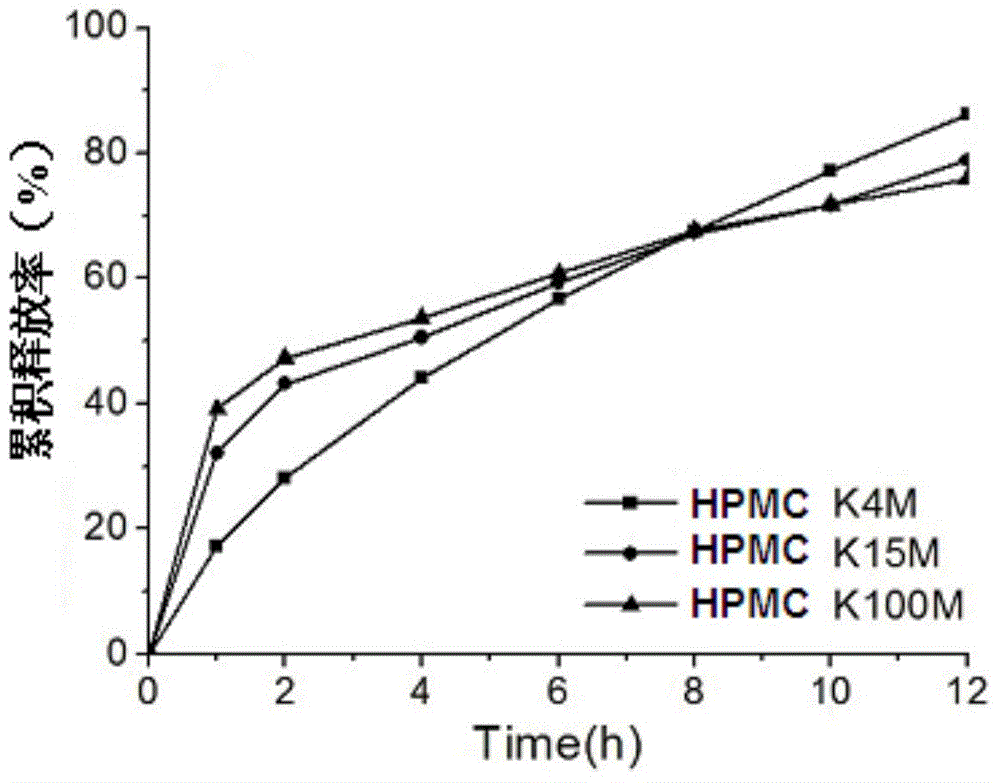
[0029] 1. Skeleton material screening
[0030] Under the condition that other conditions remained unchanged, the effects of HPMC with different molecular weights (ie K4M, K15M and K100M) as matrix materials on the in vitro release of ppridinol mesylate matrix sustained-release tablets were investigated. see results figure 1 , HPMC K15M and HPMC K100M as the framework materials have obvious burst release phenomenon in the early stage, but the late release is very slow, and the effect of releasing more than 80% at 12 hours is not achieved. Relatively speaking, it is better to choose HPMC K4M as the fra...
Embodiment 2
[0047] Embodiment 2, the preparation of pridinol mesylate matrix sustained-release tablet
[0048] The optimal prescription screened out by Example 1 is used to prepare pridinol mesylate matrix sustained-release tablets, and the preparation method is as follows:
[0049] a. Raw material pretreatment: dry the starch at 100-105°C to keep the water content below 8%; pulverize pridinol mesylate, HPMCK4M, EC, starch, CMC-Na and magnesium stearate through 100 meshes respectively screen;
[0050] b. Preparation: Take the prescribed amount of HPMC K4M, starch, EC and CMC-Na, mix well, add the prescribed amount of pridinol mesylate by equal increment method, mix well, add 50% (v / v) ethanol Make soft material from solution, make wet granules with 24-mesh sieve, dry at 50°C for 50 minutes, pass through 24-mesh sieve for granulation, then add the prescribed amount of magnesium stearate, mix well, and compress with 35N pressure to obtain primidyl mesylate Nuo matrix sustained-release tab...
Embodiment 3
[0053] Embodiment 3, the in vitro release degree investigation of pridinol mesylate matrix sustained-release tablet
[0054] Take commercially available pridinol mesylate ordinary tablets and 6 tablets of pridinol mesylate matrix sustained-release tablets prepared in Example 2 respectively, and measure the cumulative release rate. see results Figure 6 , compared with ordinary pridinol mesylate tablets, the pridinol mesylate matrix sustained-release tablets prepared by the present invention have obvious sustained-release effects.
[0055] Get respectively 3 batches of pridinol mesylate matrix sustained-release tablets prepared in Example 2, and measure its cumulative release rate. see results Figure 7 , 3 batches of pridinol mesylate matrix sustained-release tablets have regular drug release in vitro, which shows that the prescription and process of pridinol mesylate matrix sustained-release tablets of the present invention are reasonable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com