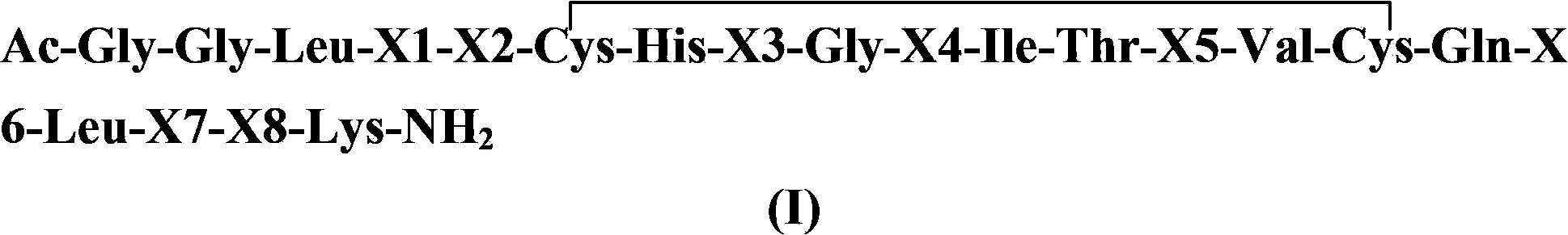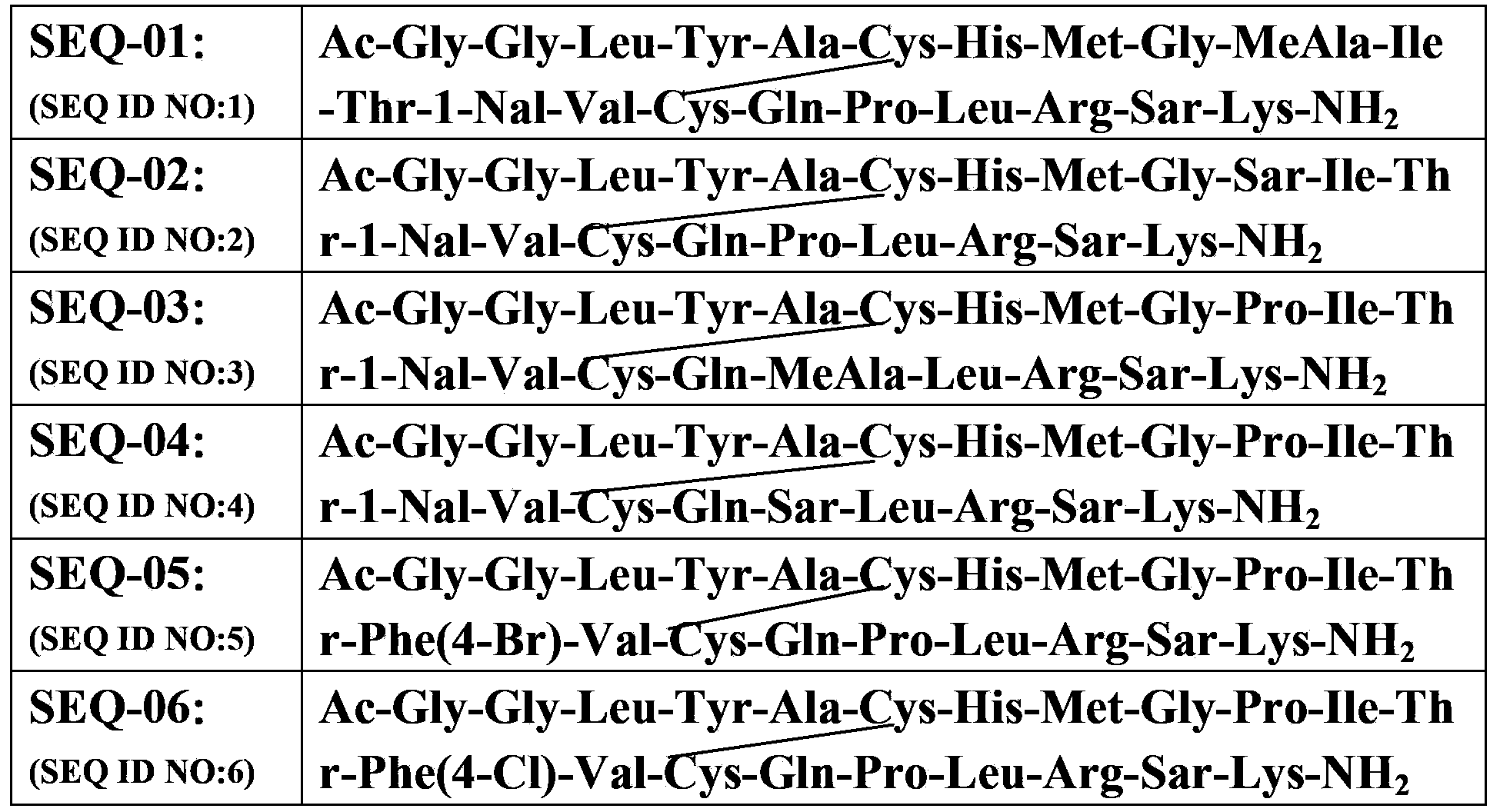Mimetic peptide of erythropoietin, preparation method and applications thereof
A technology of mimicking peptides and medicinal salts, which is applied in the field of erythropoietin mimicking peptides, and can solve problems such as poor stability in vivo and inability to meet the conditions for drug production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
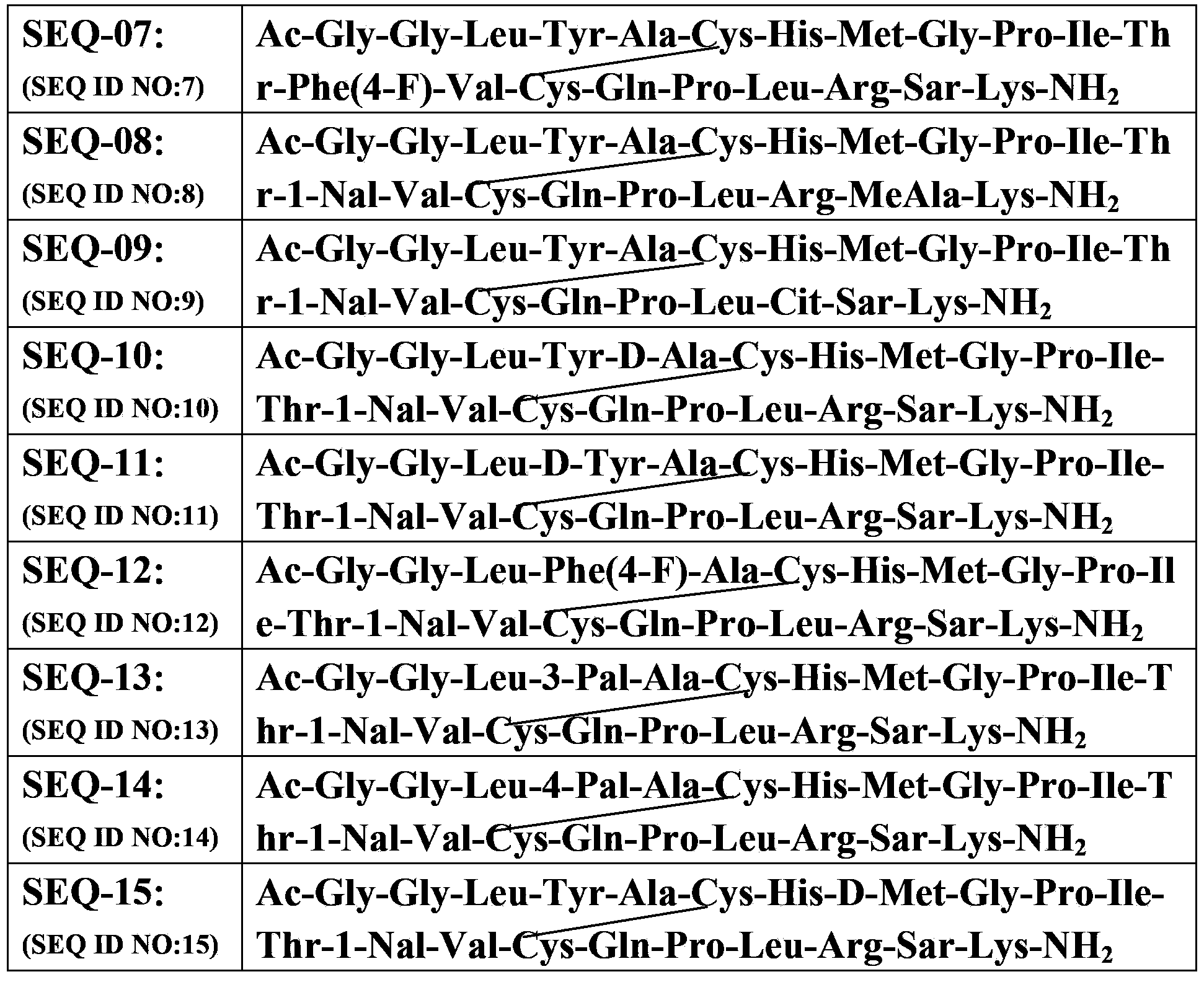
[0093] Example 1: Synthesis of SEQ-01 (see Table 1 for the structural sequence)
[0094] With 1.0g Rink-amide resin (0.25mmol) as a solid phase carrier, with Fmoc-Ala-OH, Fmoc-Arg(pbf)-OH, Fmoc-Cys(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Gly-OH, Fmoc-His(Trt)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-Lys(Boc)-OH, Fmoc-Met-OH, Fmoc-MeAla-OH, Fmoc- 1-Nal-OH, Fmoc-Phe-OH, Fmoc-Pro-OH, Fmoc-Ser(tBu)-OH, Fmoc-Sar-OH, Fmoc-Val-OH as raw material, HBTU-HOBt as condensing agent, according to SEQ According to the amino acid sequence of -01, the peptide resin was synthesized according to the standard Fmoc solid-phase peptide synthesis method. Use 20ml of trifluoroacetic acid: thioanisole: m-cresol: ethanedithiol: water (8.25:0.5:0.5:0.25:0.5, volume ratio) as the lysate, react at 0°C for 30 minutes, and at room temperature for 90 minutes, The peptide is deprotected and cleaved from the resin. Crude peptide dissolved in 20% DMSO / H 2 O solution is used as a medium for oxidation to form a disulfide ...
Embodiment 2
[0095] Example 2: Synthesis of SEQ-02 (see Table 1 for the structural sequence)
[0096] With 1.0g Rink-amide resin (0.25mmol) as a solid phase carrier, with Fmoc-Ala-OH, Fmoc-Arg(pbf)-OH, Fmoc-Cys(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Gly-OH, Fmoc-His(Trt)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-Lys(Boc)-OH, Fmoc-Met-OH, Fmoc-1-Nal-OH, Fmoc-Phe-OH, Fmoc-Pro-OH, Fmoc-Ser(tBu)-OH, Fmoc-Sar-OH, Fmoc-Tyr(OtBu)-OH, Fmoc-Val-OH as raw material, HBTU-HOBt as condensing agent According to the amino acid sequence of SEQ-02, according to the method of Example 1, SEQ-02 was obtained. The results of MALDI-Tof mass spectrometry are shown in Table 1.
Embodiment 3
[0097] Example 3: Synthesis of SEQ-03 (see Table 1 for the structural sequence)
[0098] With 1.0g Rink-amide resin (0.25mmol) as a solid phase carrier, with Fmoc-Ala-OH, Fmoc-Arg(pbf)-OH, Fmoc-Cys(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Gly-OH, Fmoc-His(Trt)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-Lys(Boc)-OH, Fmoc-MeAla-OH, Fmoc-Met-OH, Fmoc- 1-Nal-OH, Fmoc-Phe-OH, Fmoc-Pro-OH, Fmoc-Ser(tBu)-OH, Fmoc-Sar-OH, Fmoc-Tyr(OtBu)-OH, Fmoc-Val-OH as raw materials, HBTU-HOBt is a condensing agent. According to the amino acid sequence of SEQ-03, according to the method of Example 1, SEQ-03 was obtained. MALDI-Tof mass spectrometry analysis results Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com