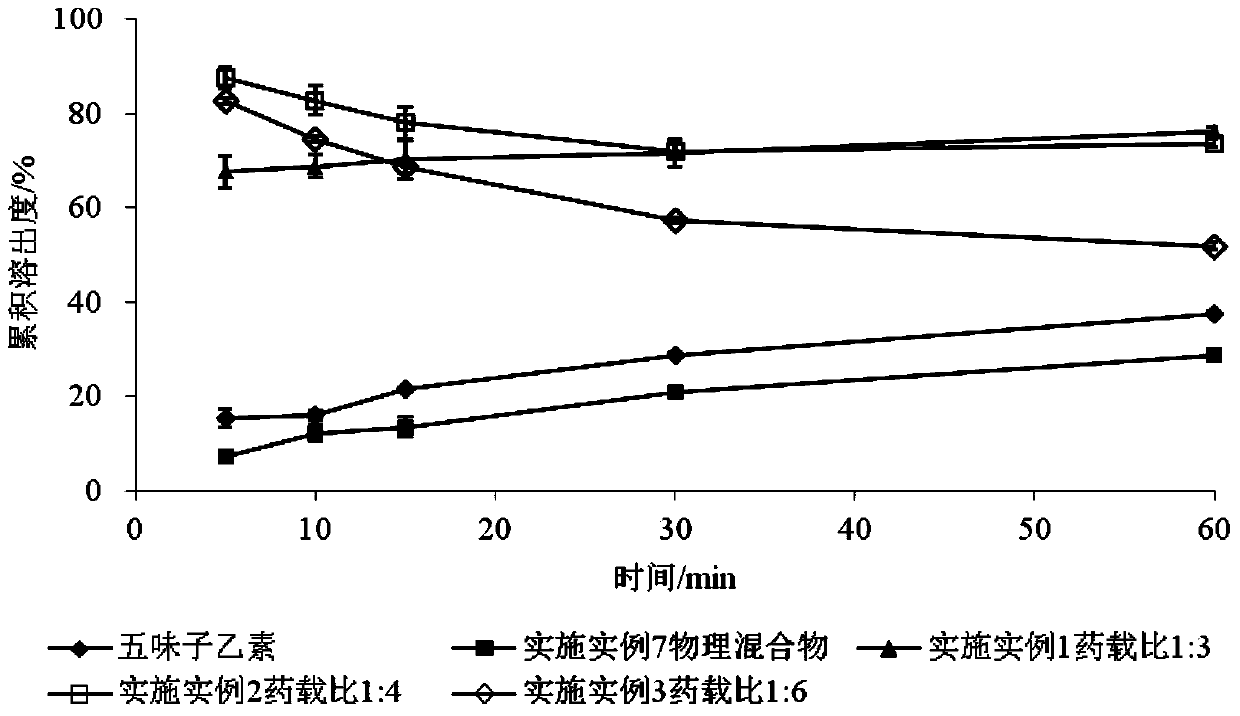
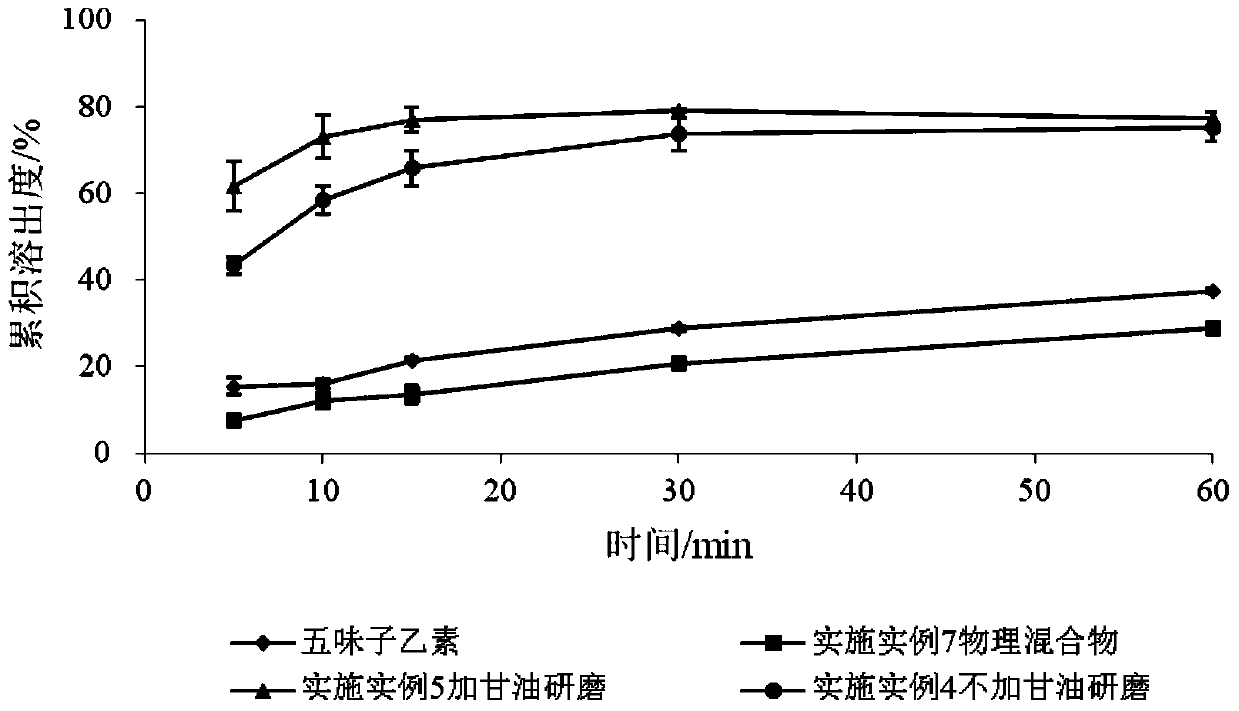
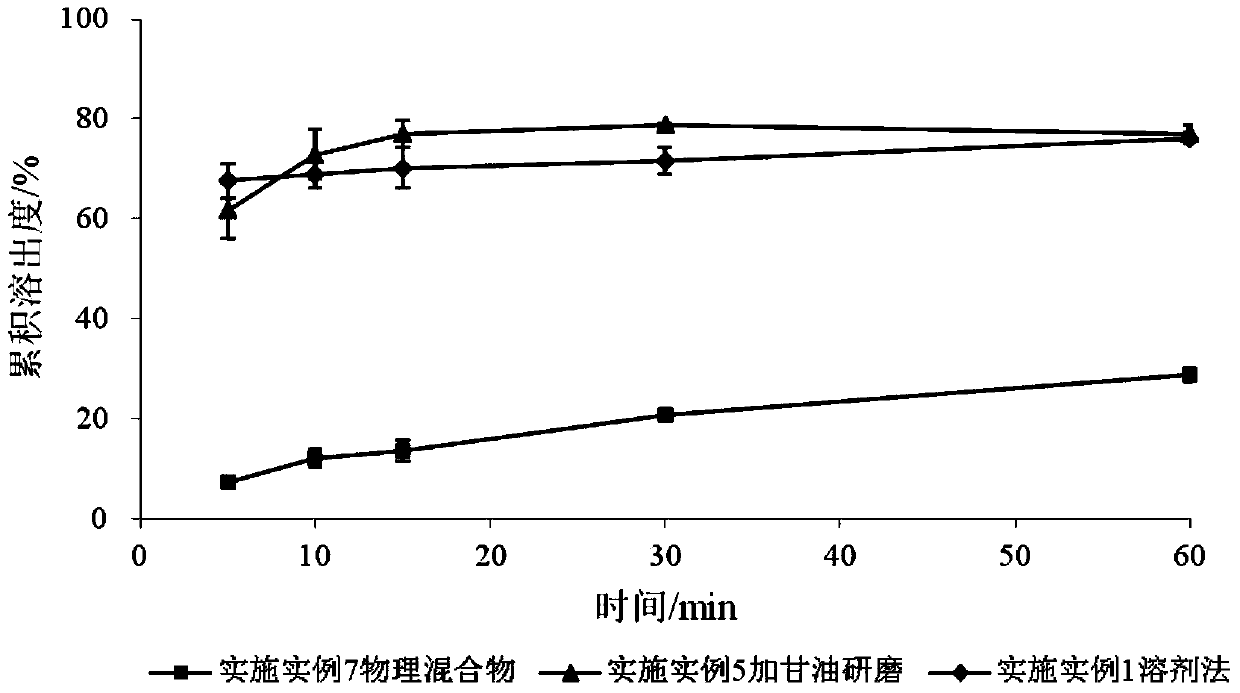
SchisandrinB solid dispersion as well as preparation method and preparation thereof
A technology of schisandrin B and solid dispersion, which is applied in the directions of non-active ingredients, such as medical preparations, pharmaceutical formulations, and active ingredients of heterocyclic compounds, can solve the problem that patients take a large dose, and there is no literature report to increase the solubility of schisandra B. Dissolution, increase side effects and other problems, to achieve the effect of improving in vitro dissolution, improving bioavailability and formulation stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Solvent method prepares Schizandrin B / PVP solid dispersion
[0027] Measure 500ml of ethanol, add 15g of povidone K90 into it, fully shake and dissolve. Then add 5 g of schisandrin B, add in a rotary evaporator, concentrate until the solvent is basically volatilized, quench, and dry, and the obtained solid is pulverized and passed through a 80-mesh sieve to obtain the solid dispersion of the present invention.
Embodiment 2
[0028] Embodiment 2: Solvent method prepares Schizandrin B / PVP solid dispersion
[0029] Measure 500ml of ethanol, add 20g of povidone K90 into it, fully shake and dissolve. Then add 5 g of schisandrin B, add in a rotary evaporator, concentrate until the solvent is basically volatilized, quench, and dry, and the obtained solid is pulverized and passed through a 80-mesh sieve to obtain the solid dispersion of the present invention.
Embodiment 3
[0030] Embodiment 3: Solvent method prepares Schizandrin B / PVP solid dispersion
[0031] Measure 500ml of ethanol, add 30g of povidone K90 into it, fully shake and dissolve. Then add 5 g of schisandrin B, add in a rotary evaporator, concentrate until the solvent is basically volatilized, quench, and dry, and the obtained solid is pulverized and passed through a 80-mesh sieve to obtain the solid dispersion of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com