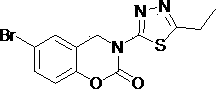
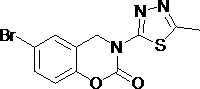
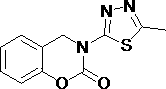
3-(1,3,4- thiadiazole)-1,3-benzooxazine-2-acetone compounds and application thereof
A ketone compound and thiadiazolyl-based technology, which is applied in the field of pesticides, can solve problems that have not been reported, and achieve good bactericidal activity, easy-to-obtain materials, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: 3-(5-Methyl-1,3,4-thiadiazol-2-yl)-3,4-dihydro -2H- Synthesis of 1,3-Benzoxazin-2-ones.
[0023] Add 2.26 mmol 2-((5-methyl-1,3,4-thiadiazol-2-yl)aminomethyl)phenol into a 100 mL round bottom flask, heat to dissolve with 35 mL toluene, pour into the flask Add 6.78 mmol of triethylamine, weigh 1.13 mmol of triphosgene and dissolve it in toluene, add the toluene solution of triphosgene dropwise in an ice bath, after the dropwise addition, heat and reflux at 95°C for 2 h (TLC tracking detection), after cooling, Add an appropriate amount of ethyl acetate to the reaction system, filter with suction, wash the filter cake with ethyl acetate, and purify by column chromatography to obtain a white solid with a yield of 56.5% and a melting point (mp): 212.1-212.4 °C.
[0024] 1 H NMR (CDCl 3 , 500 MHz) δ: 7.35(t, J=7.5, 1H), 7.29(t, J=7.5, 1H), 7.23(t, J=7.5, 1H), 7.14(d, J=8.5, 1H), 5.354(s, 2H), 2.717(s, 3H).
[0025] 13 C NMR ( CDCl 3 , 125 MHz), δ: 163.23, 1...
Embodiment 2
[0027] Example 2: 3-(5-Propyl-1,3,4-thiadiazol-2-yl)-3,4-dihydro -2H- Synthesis of 1,3-benzoxazin-2-one.
[0028] Add 3 mmol of 2-((5-propyl-1,3,4-thiadiazol-2-yl)aminomethyl)phenol into a 100 mL round bottom flask, heat to dissolve with 35 mL of toluene, pour into the flask Add 9 mmol of triethylamine, weigh 1.5 mmol of triphosgene and dissolve it in toluene, add the toluene solution of triphosgene dropwise in an ice bath, after the dropwise addition, heat and reflux at 95°C for 2 h (TLC tracking detection), after cooling, Add an appropriate amount of ethyl acetate to the reaction system, filter with suction, wash the filter cake with ethyl acetate, and purify it by column chromatography to obtain a white solid with a yield of 20.8% and a melting point (mp): 178.2-178.4 °C.
[0029] 1 H NMR (CDCl 3 , 500 MHz) δ: 735(t, 1H), 7.3(d, J=7.0, 1H), 7.21~7.24(m, 1H), 7.13(d, J=8.0, 1H), 5.35(s, 2H ), 3.01(t, J=7.5, 2H ), 1.80~1.88(m, 2H ), 1.04(t, 3H).
[0030] 13 C NMR (C...
Embodiment 3
[0032] Example 3: 3-(5-isopropyl-1,3,4-thiadiazol-2-yl)-3,4-dihydro -2H- Synthesis of 1,3-Benzoxazin-2-ones.
[0033] Add 2 mmol of 2-((5-propyl-1,3,4-thiadiazol-2-yl)aminomethyl)phenol into a 100 mL round bottom flask, heat to dissolve with 35 mL of toluene, pour into the flask Add 6 mmol of triethylamine, weigh 1 mmol of triphosgene and dissolve it in toluene, add the toluene solution of triphosgene dropwise in an ice bath, after the dropwise addition, heat and reflux at 95 °C for 2 h (TLC tracking detection), after cooling, Add an appropriate amount of ethyl acetate to the reaction system, filter with suction, wash the filter cake with ethyl acetate, and purify it by column chromatography to obtain a white solid with a yield of 71.70% and a melting point (mp): 159.8-161.0 °C.
[0034] 1 H NMR (CDCl 3 , 500 MHz) δ: 7.34(s, 1H), 7.30(t, 1H), 7.21~7.25(m, 1H), 7.12(t, 1H), 5.34(s, 2H), 3.38~3.41(m, 1H ), 1.44~1.46(m, 6H).
[0035] 13 C NMR (CDCl 3 , 125 MHz) δ: 174.22...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com