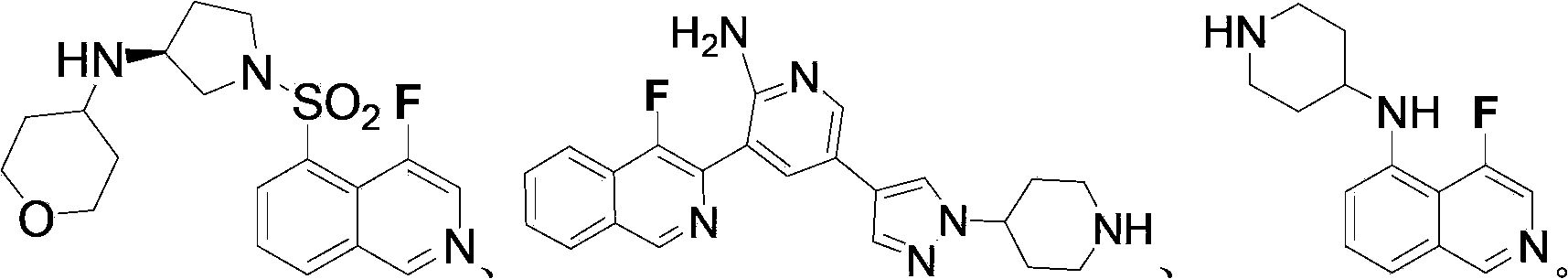
Fluorinated isoquinoline compounds and preparation method thereof
A technology for isoquinolines and compounds, which is applied in the field of fluoroisoquinoline compounds and their preparation, can solve the problems of harsh conditions, cumbersome routes, and few methods for fluoroisoquinoline compounds, and achieve low cost and low substrate good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
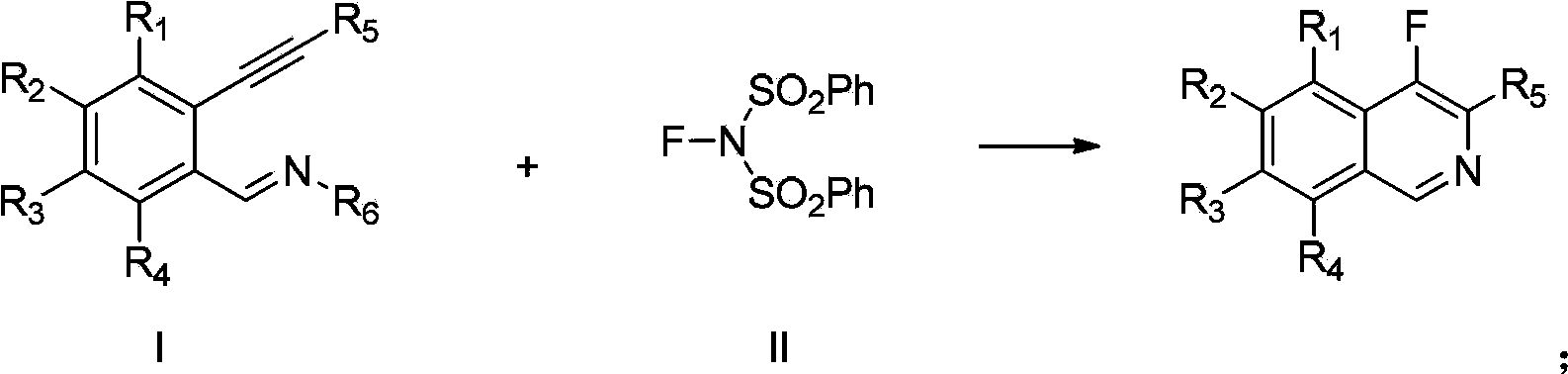
Image
Examples
Embodiment 1
[0028] Example 1: preparation of
[0029] 3.4mg (0.02mmol) of AgNO 3 , 7.4mg (0.1mmol) of Li 2 CO 3 , 47.5mg (0.15mmol) of N-fluorobisbenzenesulfonamide (NFSI) was added to the reaction tube, 1.5mL of N,N-dimethylacetamide was added, and then 24.1mg (0.1mmol) of alkyne substrate was added Stir the reaction at 30°C for 5 hours; add water, extract with diethyl ether, combine the extracts, concentrate, column chromatography, and elute with ethyl acetate and petroleum ether gradient to obtain 17.7 mg of the product The yield was 87%.
[0030] 1 H NMR (400MHz, CDCl 3 ):9.07(s,1H),8.03(d,J=8.4Hz,1H),7.94(d,J=8.4Hz,1H),7.71(dd,J=7.2,7.2Hz,1H),7.57(dd ,J=7.2,7.2Hz,1H),3.00(dt,J=8.0,2.4Hz,2H),1.79(tt,J=8.0,7.6Hz,2H),1.43(tq,J=7.6,7.6Hz, 2H),0.96(q,J=7.6Hz,3H);
[0031] 13 C NMR (100MHz, CDCl 3 ): 152.2(d, J=255.7Hz), 147.2(d, J=6.1Hz), 140.7(d, J=16.0Hz), 130.3, 128.8(d, J=2.3Hz), 126.9, 126.8(d, J=2.3Hz), 126.5(d, J=16.7Hz), 119.3(d, J=4.5Hz), 31.2, 30.7, 22.5, 13.9;
...
Embodiment 2
[0034] Example 2: preparation of
[0035] 3.4mg (0.02mmol) of AgNO 3 , 7.4mg (0.1mmol) of Li 2 CO 3 , 47.5mg (0.15mmol) of N-fluorobisbenzenesulfonamide (NFSI) was added to the reaction tube, 1.5mL of N,N-dimethylacetamide was added, and then 26.1mg (0.1mmol) of alkyne substrate was added Stir the reaction at 30°C for 6 hours; add water, extract with ether, combine the extracts, concentrate, column chromatography, and elute with ethyl acetate and petroleum ether gradient to obtain 19.4 mg of the product The yield was 87%.
[0036] 1 H NMR (400MHz, CDCl 3 ):9.13(s,1H),8.12(d,J=8.4Hz,1H),8.10(d,J=8.4Hz,2H),7.97(d,J=8.4Hz,1H),7.73(dd,J =8.0,7.2Hz,1H),7.61(t,J=7.2Hz,1H),7.52(d,J=7.2Hz,2H),7.42(t,J=7.2Hz,1H);
[0037] 13 C NMR (100MHz, CDCl 3 ): 132.3(d, J=262.5Hz), 147.6(d, J=6.1Hz), 136.6(d, J=10.6Hz), 135.7(d, J=5.4Hz), 130.6(d, J=1.5Hz ),,129.4(d,J=2.3Hz),128.9(d,J=6.0Hz),128.5,128.4,127.8,127.3(d,J=16.7Hz),126.9(d,J=1.5Hz),119.9 (d, J=5.3Hz);
[0038] 19 F NMR...
Embodiment 3
[0040] Example 3: preparation of
[0041] 3.4mg (0.02mmol) of AgNO 3 , 7.4mg (0.1mmol) of Li 2 CO 3 , 95.0mg (0.15mmol) of N-fluorobisbenzenesulfonamide (NFSI) was added to the reaction tube, 1.5mL of N,N-dimethylacetamide was added, and then 22.5mg (0.1mmol) of alkyne substrate was added Stir the reaction at 60°C for 7 hours; add water, extract with ether, combine the extracts, concentrate, column chromatography, and elute with ethyl acetate and petroleum ether gradient to obtain 10.5 mg of the product The yield was 56%.
[0042] 1 H NMR (400MHz, CDCl 3 ):8.91(s,1H),8.00(d,J=8.4Hz,1H),7.90(d,J=8.0Hz,1H),7.70(dd,J=8.4,8.0Hz,1H),7.53(dd ,J=8.0,8.0Hz,1H),2.54-2.43(m,1H),1.22-1.15(m,2H),1.09-1.01(m,2H);
[0043] 13 C NMR (100MHz, CDCl 3 ): 153.7(d, J=254.3Hz), 147.3(d, J=5.9Hz), 140.8(d, J=14.1Hz), 130.4, 128.1(d, J=2.2Hz), 126.9(d, J=2.2Hz), 126.9(d, J= 2.2Hz), 126.5, 126.2(d, J=16.4Hz), 118.8(d, J=4.5Hz), 9.9, 8.5;
[0044] 19 F NMR (376MHz, CDCl 3 ):.-145.3(s)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com