Method for manufacturing tolylene diisocyanate
A technology of toluene diisocyanate and its manufacturing method, which is applied to the preparation of carbamate, isocyanic acid derivatives, carbamic acid derivatives, etc., and can solve the problems of device corrosion, cumbersome handling, and strong toxicity of phosgene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
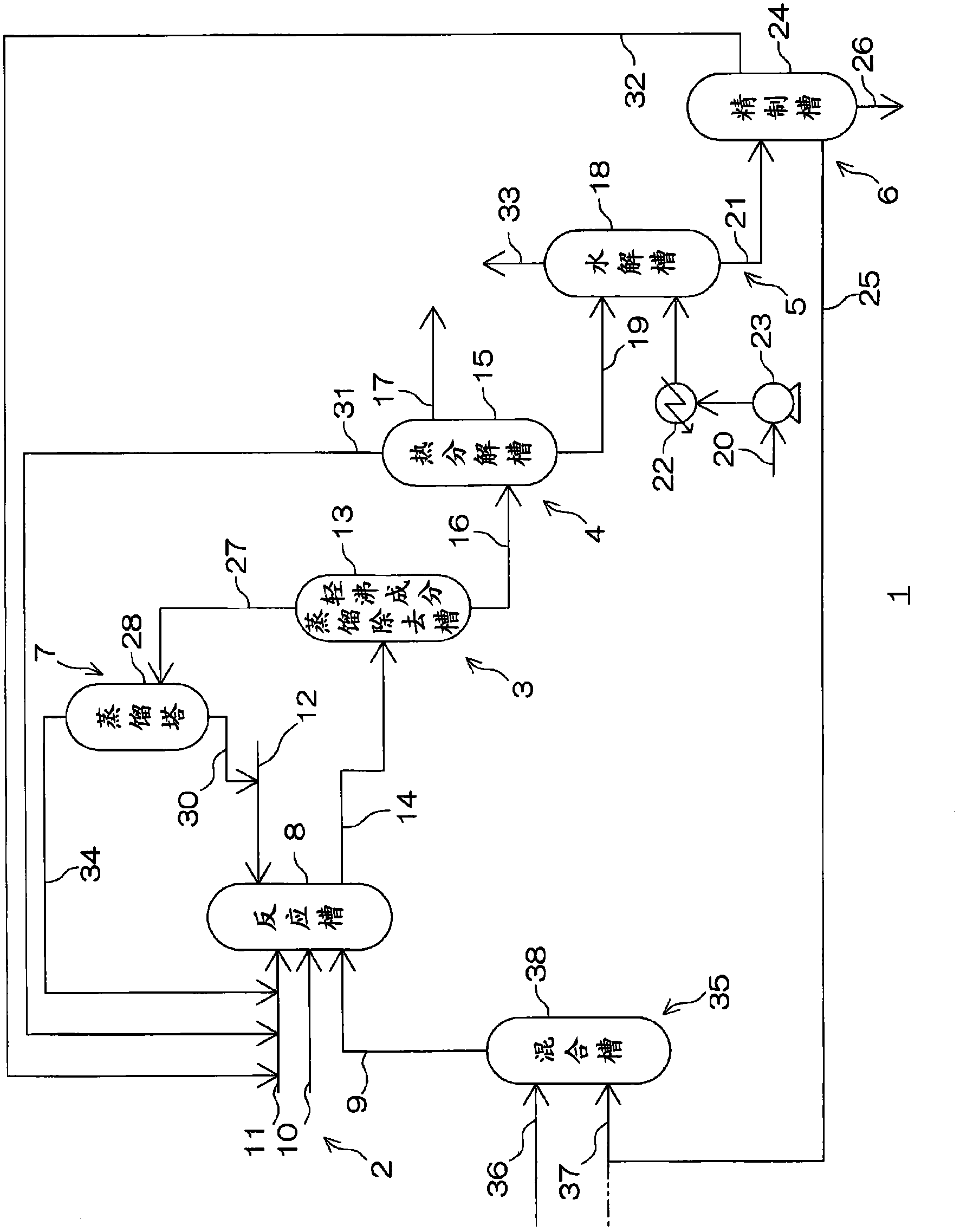
Image
Examples
manufacture example 1
[0224] (Urethane Manufacturing Process)
[0225] Put the first diaminotoluene (2,4-diaminotoluene / 2,6-diaminotoluene= 80 / 20 (molar ratio)) (122g: 1mol), butyl carbamate (333g: 2.85mol) and 1-butanol (211g: 2.85mol), zinc p-toluenesulfonate (1.0g: 2.5mmol) as a catalyst ), while feeding nitrogen with a flow rate of 1 L per minute and stirring at 500 rpm, while adjusting the internal pressure with a pressure control valve, so that the reaction temperature remained at 200 ° C, reacted for 8 hours to obtain a reaction solution.
[0226] A part of the reaction solution was collected and quantified, and the results were confirmed. 2,4-bis(butoxycarbonylamino)toluene (2,4-toluene dicarbamate) and 2,6-bis(butoxycarbonylamino) Toluene dicarbamate was obtained at a yield of 95 mol% based on the total amount of toluene (2,6-toluene dicarbamate).
[0227] (decompression distillation removal of light boiling components)
[0228] 387.77 g of the reaction liquid obtained by the above-ment...
Embodiment 1
[0239] 100 g of the first diaminotoluene (2,4-diaminotoluene / 2,6-diaminotoluene=80 / 20 (molar ratio)) was used as the decomposed diaminotoluene obtained in Production Example 1 of the second diaminotoluene (2,4-diaminotoluene / 2,6-diaminotoluene=95 / 5 (molar ratio)) 21 g were mixed, and mixed diaminotoluene was obtained (mixing process).
[0240] The isomer ratio of the obtained mixed diaminotoluene was 2,4-diaminotoluene / 2,6-diaminotoluene=82.6 / 17.4 (molar ratio).
[0241] Except having used mixed diaminotoluene instead of the 1st diaminotoluene, it carried out similarly to manufacture example 1, and toluene dicarbamate was obtained. In addition, it was confirmed that 2,4-bis(butoxycarbonylamino)toluene (2,4-toluene dicarbamate) and 2,6-bis(butoxycarbonylamino)toluene (2, 6-toluene dicarbamate) was obtained at a yield of 95 mol % based on the total amount of toluene dicarbamate.
[0242] Moreover, except having used the obtained toluene dicarbamate, it carried out similarly to...
Embodiment 2
[0247] Toluene dicarbamate was obtained in the same manner as in Example 1, except that 27 g of the decomposed diaminotoluene (second diaminotoluene) obtained in Production Example 1 was mixed with 100 g of the first diaminotoluene. It should be noted that it was confirmed that 2,4-bis(butoxycarbonylamino)toluene (2,4-toluene dicarbamate) and 2,6-bis(butoxycarbonylamino)toluene (2, 6-toluene dicarbamate) was obtained at a yield of 95 mol % based on the total amount of toluene dicarbamate.
[0248] The isomer ratio of mixed diaminotoluene is 2,4-diaminotoluene / 2,6-diaminotoluene=83.2 / 16.8 (molar ratio).
[0249] Moreover, except having used the obtained toluene dicarbamate, it carried out similarly to Example 1, distilled off the light boiling component under reduced pressure, and thermally decomposed the obtained concentrate, and obtained toluene diisocyanate, and obtained the filter residue.
[0250] As a result of quantification by HPLC, the yield of the obtained toluene di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal degradation temperature | aaaaa | aaaaa |
| thermal degradation temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com