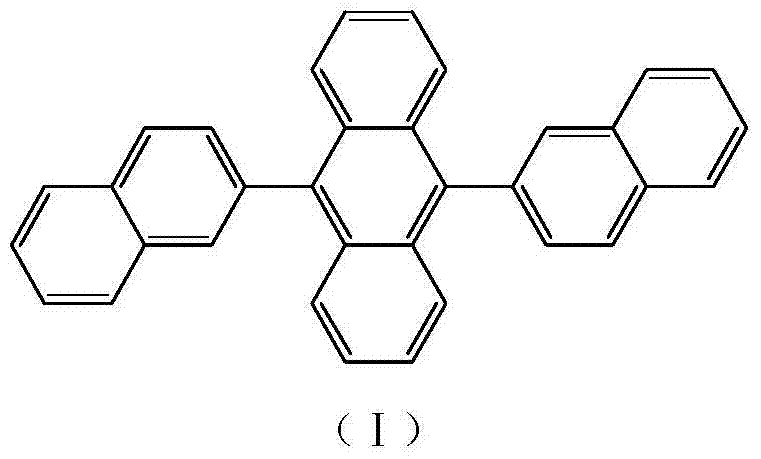
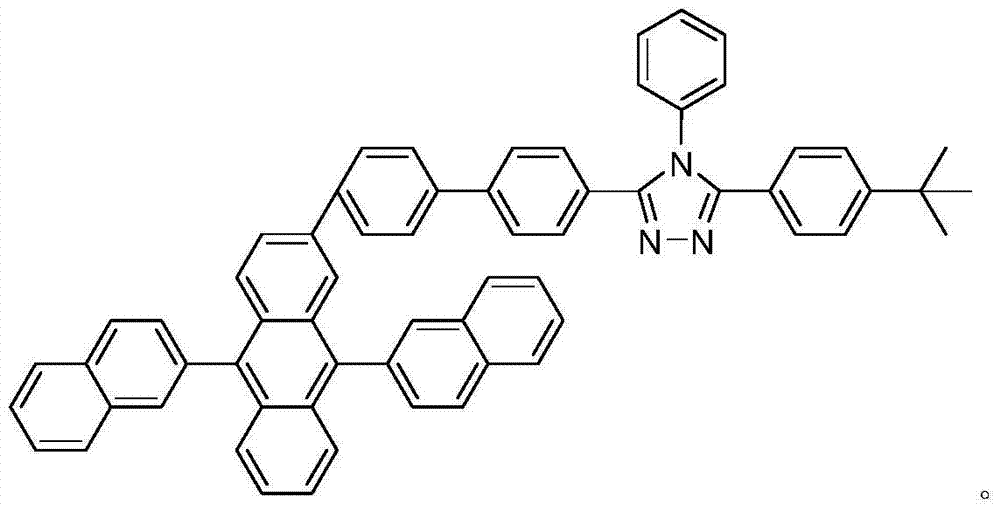
Anthracene derivative and preparation method thereof
A technology of anthracene derivatives and triazole derivatives, which is applied in the field of anthracene derivatives and their preparation, can solve the problem that the fluorescence quantum efficiency cannot meet the needs of OLED device development, and achieve the effects of enhanced conjugation and high fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: 3-(4-tert-butylphenyl)-5-(4-(2-(9,10-di(β-naphthyl))anthracenyl)biphenyl)-4-phenyl-1 , Synthesis of 2,4-triazole (β-ADN-2-TAZ):
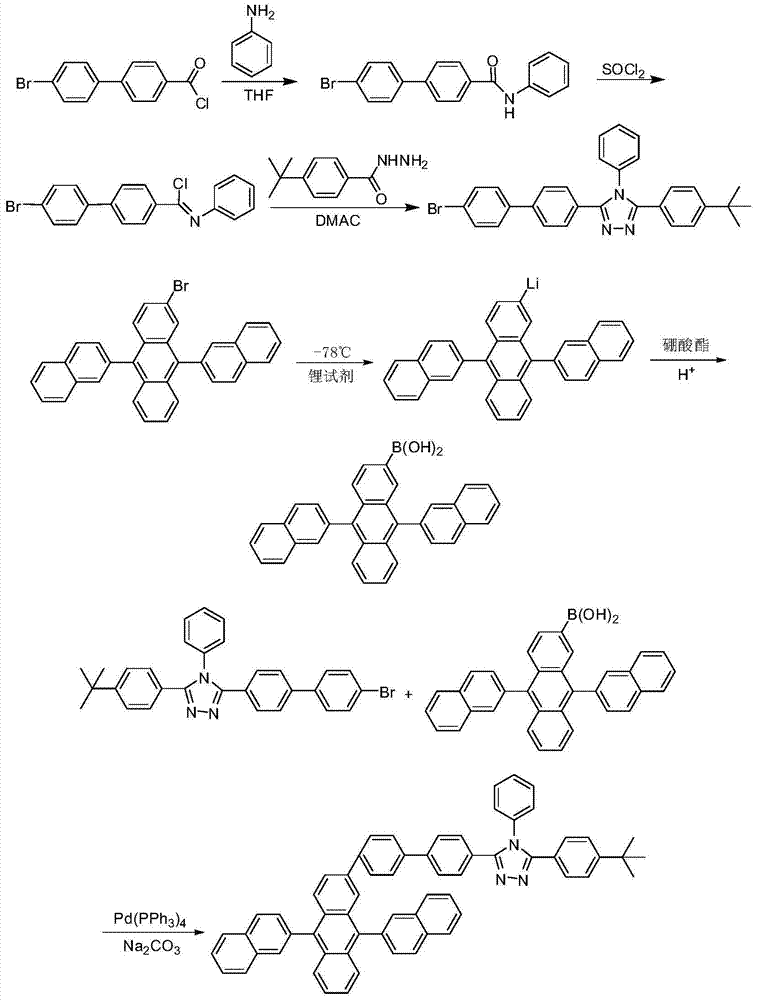
[0027] 3-(4-tert-butylphenyl)-5-(4-(2-(9,10-bis(β-naphthyl))anthracenyl)biphenyl)-4-phenyl given in this example -1,2,4-triazole (β-ADN-2-TAZ), the synthesis reaction process is as follows:
[0028]
[0029] Concrete synthetic steps are:
[0030] 1) Synthesis of 3-(4-bromobiphenyl)-5-(4-tert-butylphenyl)-4-phenyl-1,2,4-triazole
[0031] At 0°C, add p-bromobibenzoyl chloride (5.1g, 17mmol), aniline (1.7g, 18.3mmol) and 60mL of no Water tetrahydrofuran, heat preservation reaction for 3 hours, poured into water, filtered to obtain a white solid, dried to obtain N-phenyl-4-bromobiphenylcarboxamide 5.91g, yield: 96.9%, purity 98.5%. Weigh 2.75g (7.8mmol) of N-phenyl-4-bromobiphenylcarboxamide and 31g (260mmol) of thionyl chloride, and add them to a 250mL tank equipped with a polytetrafluoroethylene stirrer, a reflux condenser and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com