Perylene tetracarboxylic dianiline conjugated polymer and preparation method and application thereof
A perylenetetracarboxylic acid diimide and conjugated polymer technology, which is applied in the field of conjugated polymers and its preparation, can solve the problems of poor film-forming processability, poor solar light efficiency, and poor solubility, and achieve enhanced Effects of flatness and conjugation, wide absorption range, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
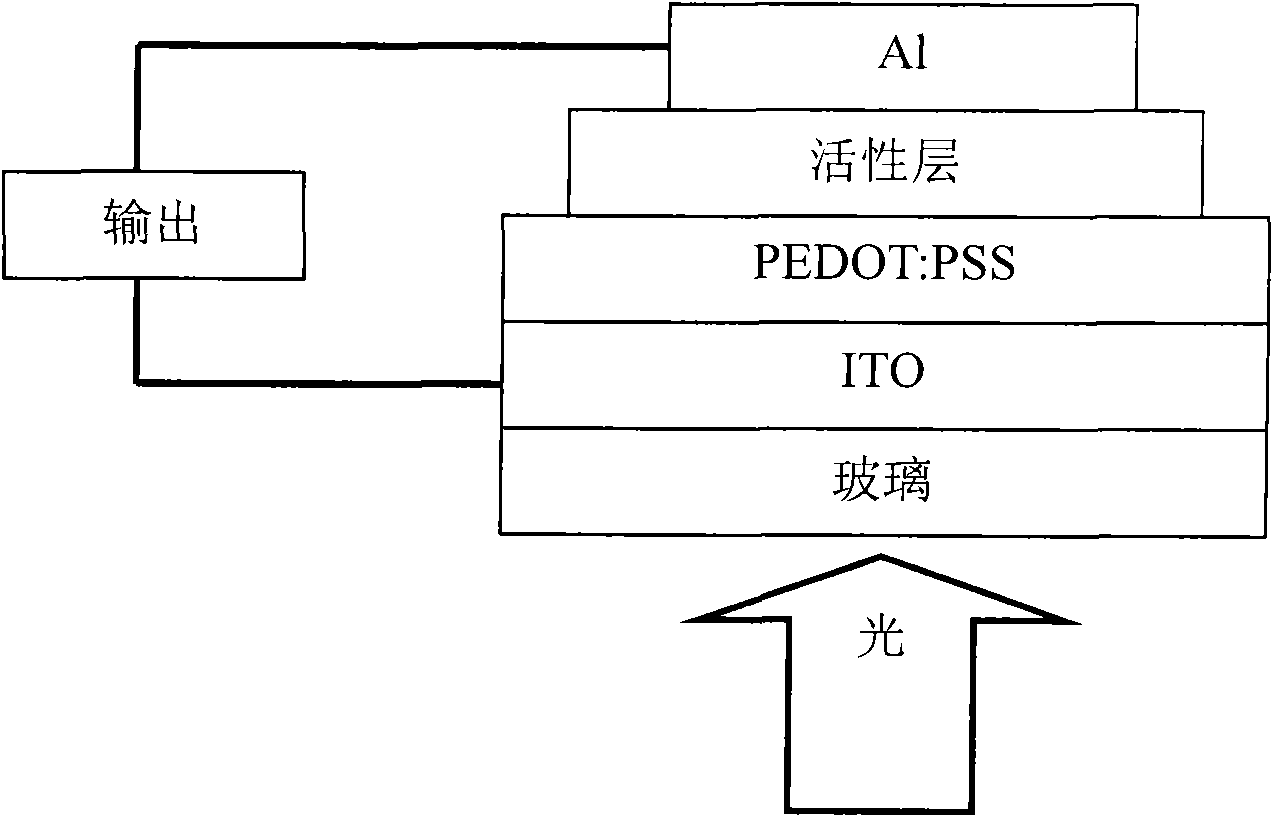
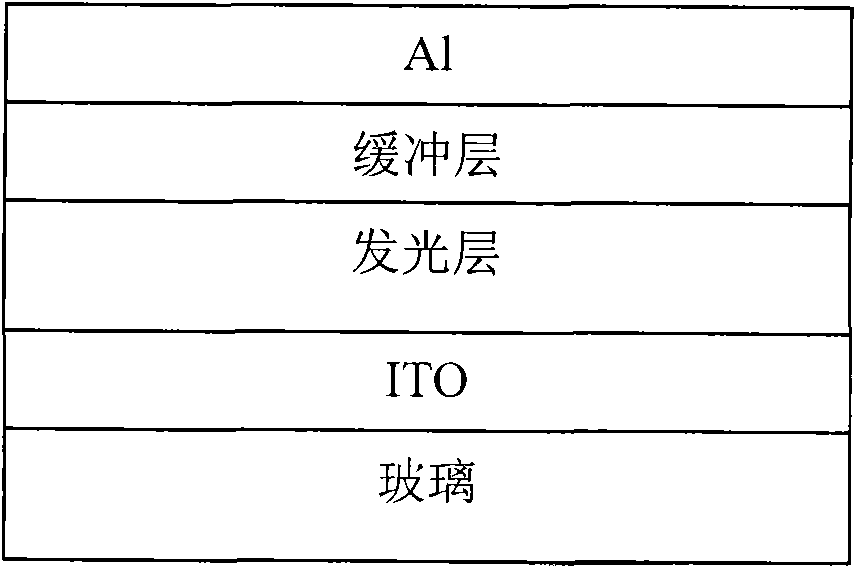
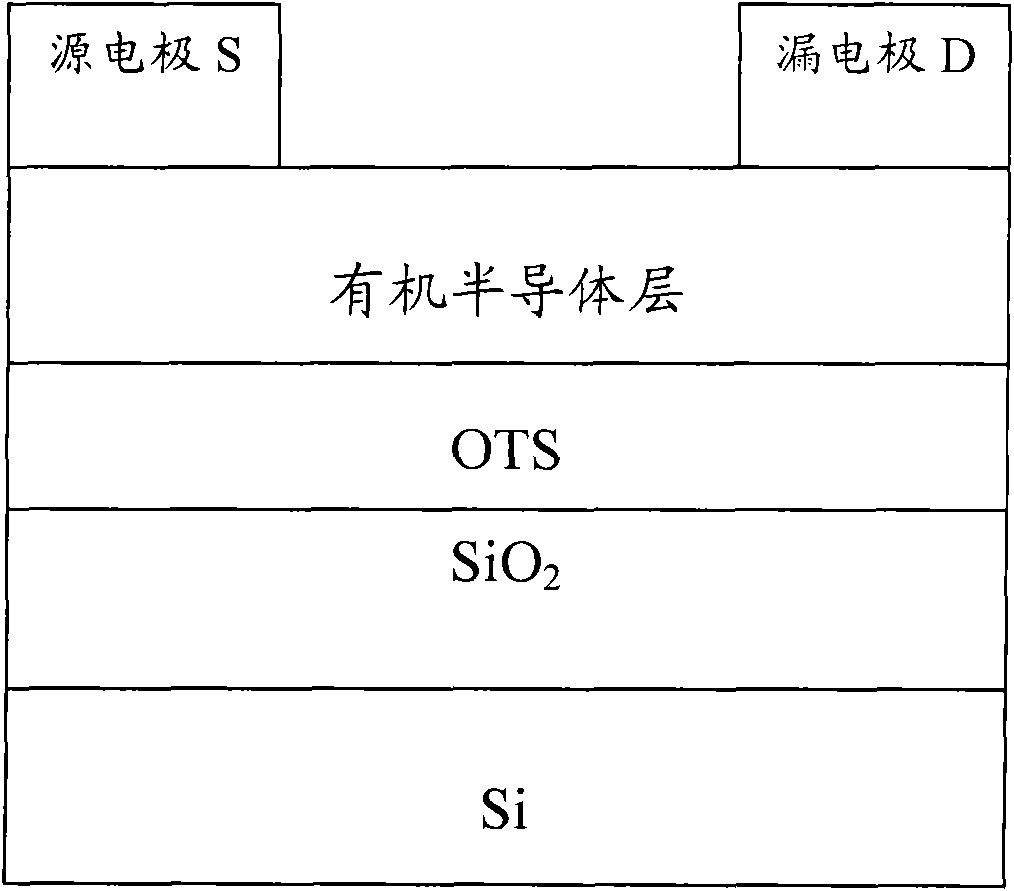
Image
Examples
Embodiment 1
[0069] About poly N,N'-bis-(3,4,5-tri-dodecyloxybenzene)-3,4,9,10-perylene diimide-4,7-bis(3,6- The preparation of dioctylthieno[3,2-b]thiophene)-2,1,3-benzothiadiazole, the chemical reaction formula is as follows:
[0070]
[0071] Under the protection of nitrogen, it contains the compound N,N'-bis-(3,4,5-tri-dodecyloxybenzene)-1,7-dibromo-3,4,9,10-perylene diacyl Imine 0.5mmol, 4,7-bis(5-tributyltin-(3,6-dioctylthieno[3,2-b]thiophene))-2,1,3-benzothiadiazole 0.5mmol Toluene (20mL) solution, bubbling for 0.5h to remove residual oxygen, then add Pd 2 (dba) 3 (0.014g, 0.015mmol) and P(o-Tol) 3 (0.0093g, 0.030mmol) and bubbling for 0.5h to remove residual oxygen, and then heated to 80°C for 48 hours; the mixed solution was added dropwise to methanol for sedimentation, filtered with suction, washed with methanol, dried, and then dissolved with toluene. Add to the aqueous solution of sodium diethyldithiocarbamate, then heat the mixture to 90°C and stir overnight; pass the organic ph...
Embodiment 2
[0073] About poly N,N'-bis-(3,4,5-tri-octoxybenzene)-3,4,9,10-perylene diimide-4,7-bis(3,6-dioctyl Preparation of thiopheno[3,2-b]thiophene)-2,1,3-benzothiadiazole, the chemical reaction formula is as follows:
[0074]
[0075] Under the protection of nitrogen, it contains the compound N,N'-bis-(3,4,5-tri-octyloxybenzene)-1,7-dibromo-3,4,9,10-perylene diimide Amine 0.5mmol, 4,7-bis(5-tributyltin-thieno[3,2-b]thiophene))-2,1,3-benzothiadiazole 0.5mmol in toluene / THF (30mL) solution, Bubble 0.5h to remove residual oxygen, then add Pd(PPh 3 ) 4 0.0069mmol, bubbling for 0.5h to remove residual oxygen, and then heating to 80°C for 72 hours. The mixed solution was added dropwise to methanol for sedimentation, filtered with suction, washed with methanol, dried, then dissolved in toluene, added to the aqueous solution of sodium diethyldithiocarbamate, and then heated to 80°C and stirred overnight. The organic phase was passed through alumina column chromatography, eluted with chloroben...
Embodiment 3
[0077] About poly N,N'-bis-(3,5-bis-eicosyloxy-4-toluene)-3,4,9,10-perylene diimide-4,7-bis(6-dec Preparation of dialkoxythieno[3,2-b]thiophene)-2,1,3-benzothiadiazole, the chemical reaction formula is as follows:
[0078]
[0079] Under the protection of nitrogen, the compound N,N'-bis-(3,5-di-eicosyloxy-4-toluene)-1,7-dibromo-3,4,9,10-perylene Imide 0.52mmol, 4,7-bis(5-tributyltin-(6-dodecyloxythieno[3,2-b]thiophene))-2,1,3-benzothiadiazole 0.5 mmol of toluene (20mL) solution, bubbling for 0.5h to remove residual oxygen, then add Pd(PPh 3 ) 2 Cl 2 0.0071mmol, bubbling for 0.5h to remove residual oxygen, and then heating to 100°C for 56 hours. The mixture was added dropwise to methanol for sedimentation, filtered with suction, washed with methanol, dried, then dissolved in toluene, and added to diethyl In an aqueous solution of sodium dithiocarbamate, the mixture was heated to 80°C and stirred overnight; the organic phase was passed through alumina column chromatography, eluted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com