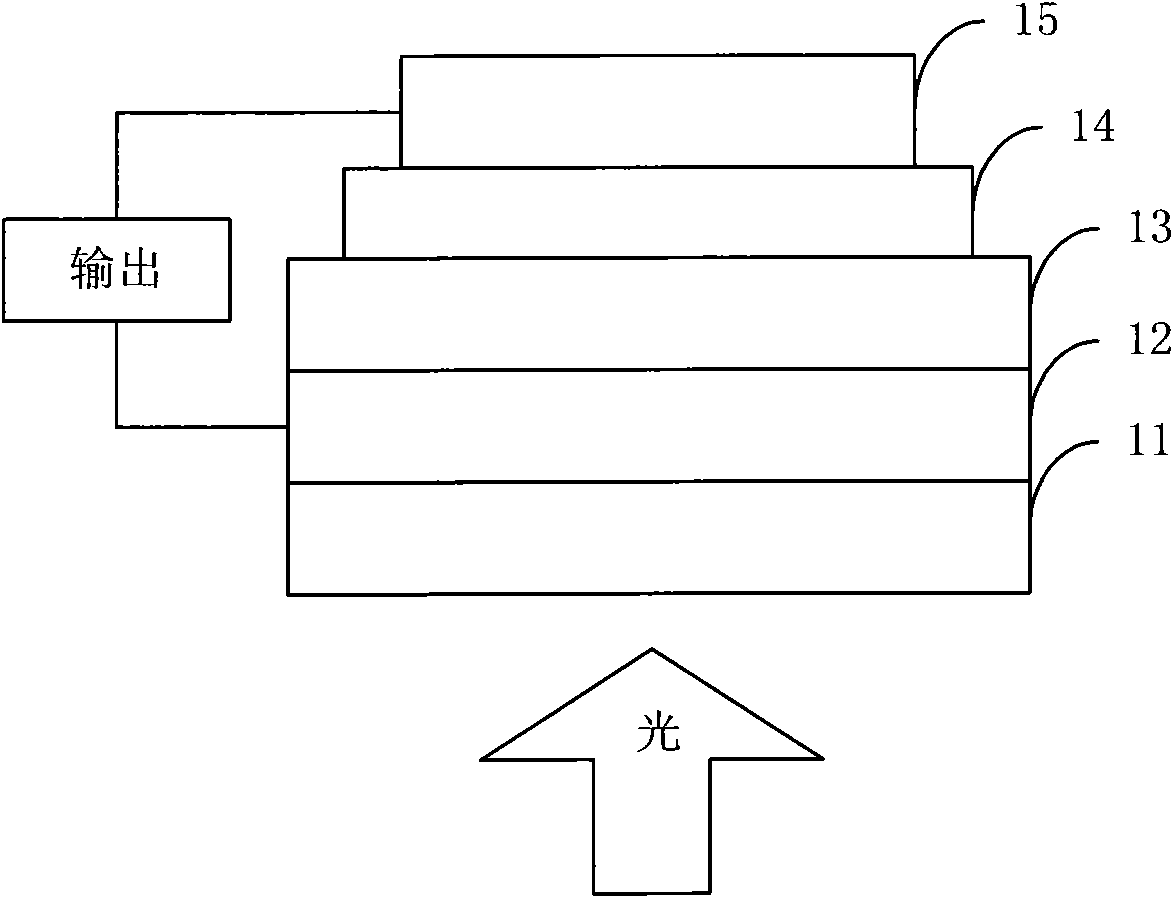
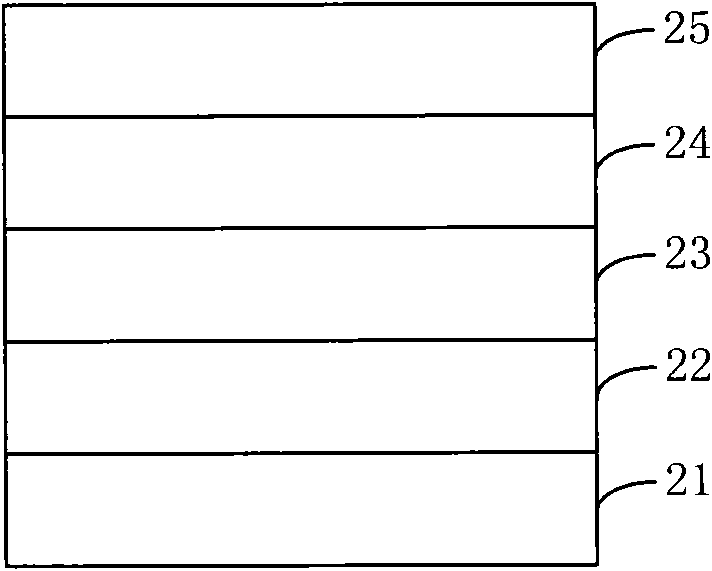
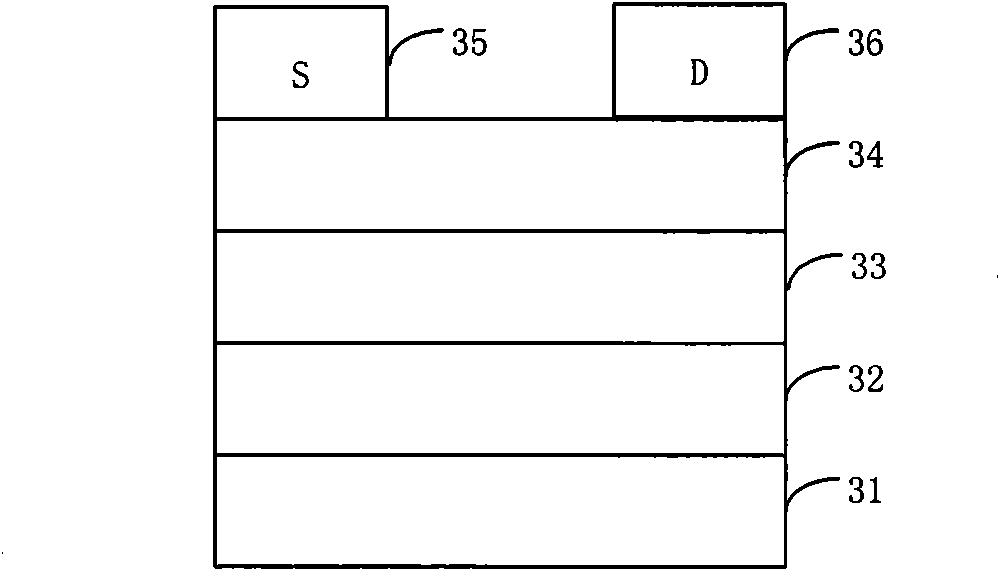
Fluorene, anthracene and 2-thiophene thiazide-containing copolymer and preparation method and application thereof
A technology of dithiophene and copolymers, which is applied in the field of copolymers, can solve problems such as limiting the scope of application, and achieve the effects of improving solubility, improving utilization, and being easy to operate and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] This embodiment discloses polymers P1 and P2 with the following structures:
[0066]
[0067] The preparation steps of P1 and P2 are as follows:
[0068] Step 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dioctylfluorene:
[0069]
[0070] Set up an anhydrous and oxygen-free reaction device, constantly stirring and N 2 Under the protection of, add 9.0mmol of white 2,7-dibromo-9,9-dioctylfluorene to the three-necked bottle, inject 150ml of refined tetrahydrofuran solvent with a syringe, and then slowly inject it with a syringe at -78℃ 27.0 mmol n-BuLi, stirred and reacted for 2 hours. After 2 hours of reaction, 30.6mmol 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborane was injected with a syringe at -78℃, and the temperature was raised to React overnight at room temperature.
[0071] After the reaction, a saturated NaCl aqueous solution was added, extracted with chloroform, dried over anhydrous sodium sulfate, filtered, and the filtrate was collected ...
Embodiment 2
[0079] This embodiment discloses polymers P3 and P4 with the following structures:
[0080]
[0081] The preparation steps of P3 and P4 are as follows:
[0082] Step 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dihexylfluorene:
[0083] The preparation process refers to step one in Example 1.
[0084] Step 3. Preparation of P3 and P4:
[0085]
[0086] Add 1 mmol, 9,10 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-dihexylfluorene in the reactor -Dibromo-2,6-bis(2-octyldecyl)anthracene 0.8mmol (refer to Klaus Mullen et al. Macromol.Chem.Phys.2006,207,1107-1115 for the synthesis method of this compound), 5,5' -Dibromo-3,3'-di-n-octyl methylene silicon-2,2'-dithiophene 0.2mmol, palladium acetate (Pd(OAc) 2 )3mg, 2mol / L Na 2 CO 3 10ml of aqueous solution and 40ml of toluene solvent, through repeated N 2 And evacuated to make the reaction system in an anaerobic state, and react at 100°C for 24h.
[0087] After reacting for 24 hours, add deionized water and t...
Embodiment 3
[0091] This embodiment discloses polymers P5 and P6 with the following structures:
[0092]
[0093] The preparation steps of P5 and P6 are as follows:
[0094] Step 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-bis(dodecyl)fluorene :
[0095] The preparation process refers to step one in Example 1.
[0096] Step 2. Preparation of P5 and P6:
[0097]
[0098] Add 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9,9-bis(dodecyl)fluorene in the reactor 1mmol, 9,10-dibromo-2-fluoroanthracene 0.5mmol (see Elimelech Rochlin et al. J.Org.Chem., 2003, 68, 216-226 for the synthesis method of this compound), 5,5'-dibromo -3,3'-Diethylhexyl methylene silicon-2,2'-bithiophene 0.5mmol, tetrakistriphenylphosphine palladium (Pd(PPh 3 ) 4 )0.02mmol, 2mol / L Na 2 CO 3 10ml of aqueous solution and 40ml of toluene solvent, through repeated N 2 And evacuated to make the reaction system in an anaerobic state, and reacted at 80℃ for 58h.
[0099] After reacting for 58 hours, add de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hole mobility | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com