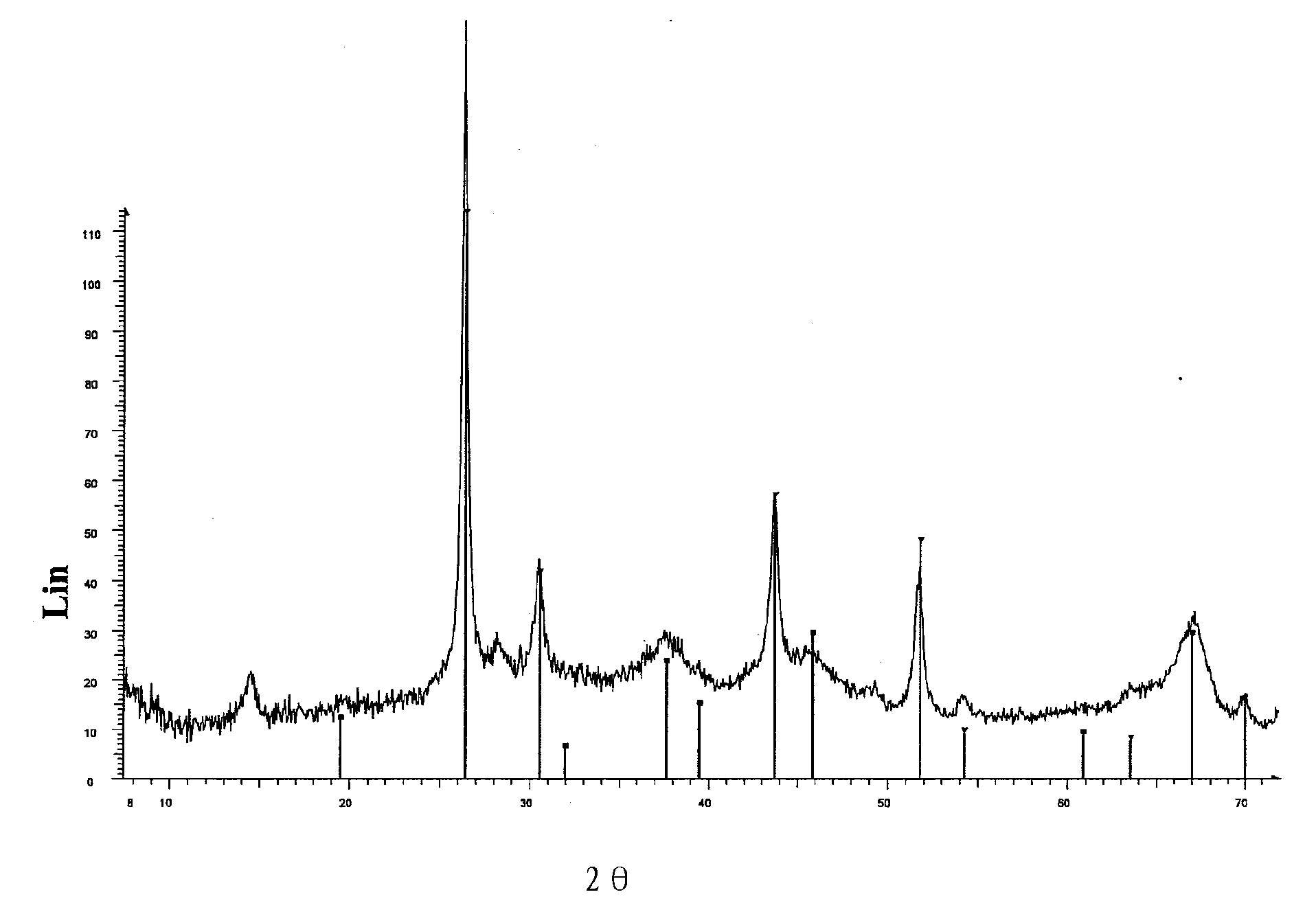
Capture mass composed of elemental sulphur deposited on porous support for capturing heavy metals
A technology of porous carrier and elemental sulfur, which is applied in the processing of synthesis gas or natural gas, and in the field of gas, which can solve the problems of entrainment of active phase and decrease of service life of capture block
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0109] Example A: Capture block M according to the invention 1 preparation of
[0110] capture block M 1 Prepared using a mixture of alumina beads and solid powdered elemental sulfur.
[0111] Description of the carrier:
[0112] The support was flashed alumina prepared by granulation with the characteristics mentioned in Table 1:
[0113] value Na 2 O (ppm) 3450 BET surface area (m2 / g) 321 V 0.004 (mL / g) 0.14 V 0.002 (mL / g) 0 V 0.01 (mL / g) 0.30 V 0.1-0.01 (mL / g) 0.10 TPV* (mL / g) 0.46 WTV** (mL / g) 0.4
[0114] Table 1
[0115] *TPV = total pore volume.
[0116] TPV was determined using the following calculations: measure particle density (Dg) using a mercury porosimeter and measure absolute density (Dab) using a helium pycnometer, then calculate TPV (mL / g) as 1 / Dg - 1 / Dab.
[0117] ** WTV = water absorption volume
[0118] WTV is determined experimentally:
[0119] Put 10 g of alumina int...
Embodiment B
[0124] Example B: Capture block M according to the invention 2 preparation of
[0125] capture block M 2 Prepared by dry impregnation of alumina beads with a solution containing a sulfur emulsion.
[0126] Description of the carrier:
[0127] The support was flashed alumina prepared by granulation with the characteristics mentioned in Table 2:
[0128] value Na 2 O (ppm) 3450 BET surface area (m2 / g) 321 V 0.004 (mL / g) 0.14 V 0.002 (mL / g) 0 V 0.01 (mL / g) 0.30 V 0.1-0.01 (mL / g) 0.10 TPV (mL / g) 0.46 WTV (mL / g) 0.4
[0129] Table 2.
[0130] Solution preparation:
[0131] Prepare the solution by mixing 60 g of sulfur in the form of an emulsion in water to obtain a solution with a volume equal to 240 mL, corresponding to the water absorption volume of 600 g of the carrier.
[0132] Preparation of capture block:
[0133] capture block M 2 Prepared by dry impregnation using 240 mL of the abo...
Embodiment C
[0135] Example C: capture block M 3 preparation (comparison)
[0136] capture block M 3 Prepared by dry impregnation of alumina beads with a solution containing a sulfur emulsion.
[0137] Description of the carrier:
[0138] The support was flashed alumina prepared by granulation with the characteristics mentioned in Table 3:
[0139] value Na 2 O (ppm) 300 BET surface area (m2 / g) 153 V 0.004 (mL / g) 0.03 V 0.002 (mL / g) 0 V 0.01 (mL / g) 0.18 V 0.1-0.01 (mL / g) 0.23 TPV (mL / g) 0.91 WTV (mL / g) 0.72
[0140] table 3.
[0141] Solution preparation:
[0142] The solution was prepared by mixing 45 g of micronized sulfur in the form of an emulsion in water to obtain a solution with a volume equal to 432 mL, corresponding to the water absorption volume of 600 g of the carrier.
[0143] Preparation of capture block:
[0144] capture block M 3 Prepared by dry impregnation by contacting 432 mL of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com