C14-position aminated derivatives of phenanthrene and indolizidine alkaloids and their preparation and anti-plant virus activity
A technology of phenanthroindolizidine and alkaloids, which is applied in the field of preparation and anti-plant virus activity, can solve the problems of difficult repetition of synthesis methods, single structure of phenanthroquinolizidine derivatives, single structure type, and the like, To achieve good anti-plant virus activity, the effect of inhibiting tobacco mosaic virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
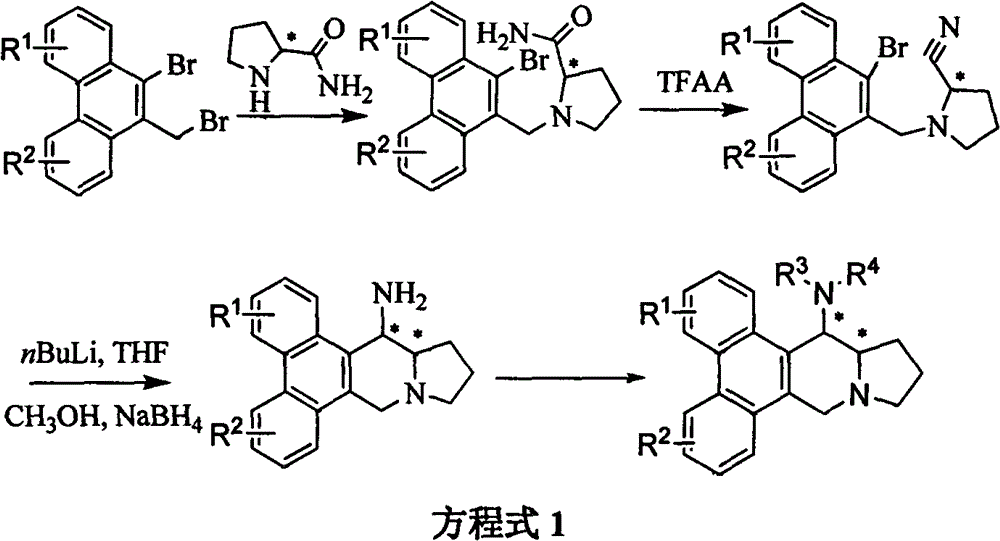
[0019] Example 1: Synthesis of 14-aminophenanthrene and indolizidine alkaloid derivatives 1-19
[0020] Synthesis of (±)-14-aminosilicate (1) and (±)-14-benzamidosilicate (10) (see Equation 3)
[0021]
[0022] Synthesis of (±)-1-(2,3,6,7-tetramethoxy-10-bromo-9-phenanthrenemethyl)pyrrole-2-carboxamide
[0023] Add 2,3,6,7-tetramethoxy-10-bromo-9-bromomethylphenanthrene (10.64mmol), (±)-pyrrole-2-carboxamide (12.77mmol) into a 250mL single-necked bottle, without Potassium carbonate in water (15.96mmol), N,N-dimethylformamide (DMF) (150mL), heated for 8 hours, desolvated, and the residue was washed with saturated brine to obtain a white solid product (±)-1-(2 , 3,6,7-tetramethoxy-10-bromo-9-phenanthrenemethyl)pyrrole-2-carboxamide (4.93g), yield 93%, melting point 238-239°C; 1 HNMR (400MHz, CDCl 3 )δ7.83(s, 1H), 7.75(s, 1H), 7.73(s, 1H), 7.64(s, 1H), 6.69(d, J=4.4Hz, 1H), 4.81(d, J=4.4 Hz, 1H), 4.62(d, J=13.2Hz, 1H), 4.48(d, J=13.2Hz, 1H, 4.12(s, 6H), 4.07(s, 3H), 4.05(s...
Embodiment 2
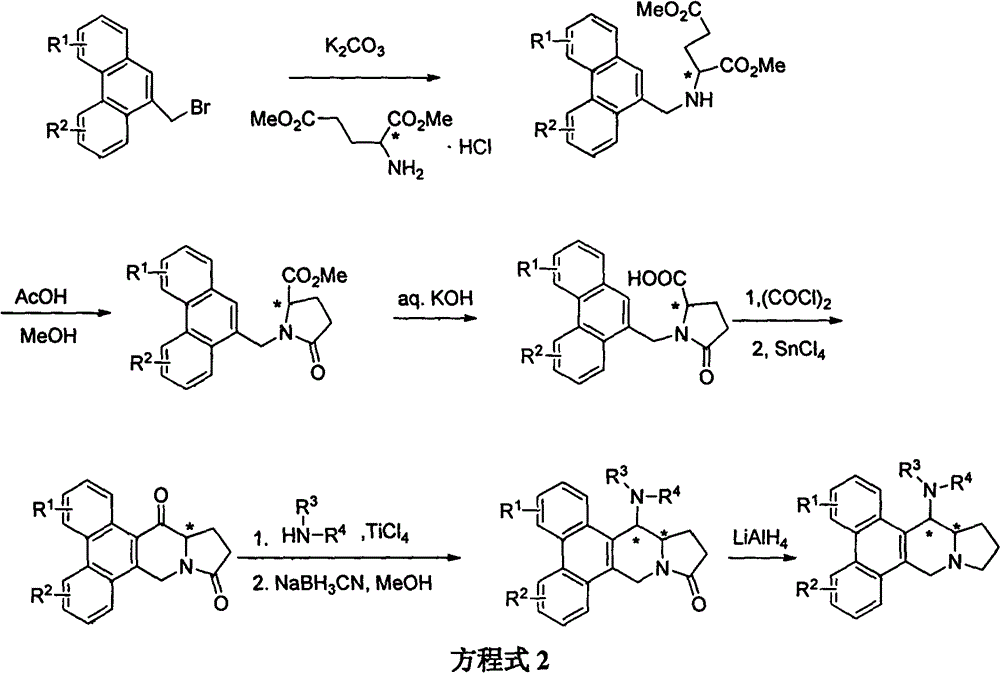
[0065] Example 2: Synthesis of 14-aminophenanthrene and indolizidine alkaloid derivatives 20-39
[0066] (13aR, 14R)-14-n-butylamine-2,3,6,7-tetramethoxyphenanthrene[9,10-b]-11-indolizidinone (28), (13aR, 14S )-14-n-butylamine-2,3,6,7-tetramethoxyphenanthrene[9,10-b]-11-indolizidone (29), (13aR, 14R)-14- Synthesis of n-butylaminosylmenine (32), (13aR, 14S)-14-n-butylaminosylphine (33) (see Equation 4)
[0067]
[0068]
[0069] Synthesis of (R)-N-(2,3,6,7-tetramethoxy-10-phenanthrenemethyl)pyroglutamic acid methyl ester
[0070] Dissolve 2,3,6,7-tetramethoxy-10-phenanthrene methyl bromide (4.0 g, 10.2 mmol) in N, N-dimethylformamide (100 mL), add D- Dimethyl glutamate hydrochloride (D-BMPAC) (2.98g, 14.1mmol) was solid. After stirring for 10 minutes, anhydrous potassium carbonate powder (2.08g) was added in one batch, and stirred at room temperature for 10 hours. N, N-dimethylformamide was distilled off under reduced pressure, dichloromethane and water were added, the...
Embodiment 3
[0107] Example 3: Research on the physicochemical properties of the C14-position aminated derivative (I) of the preferred phenanthroindolizidine alkaloid:
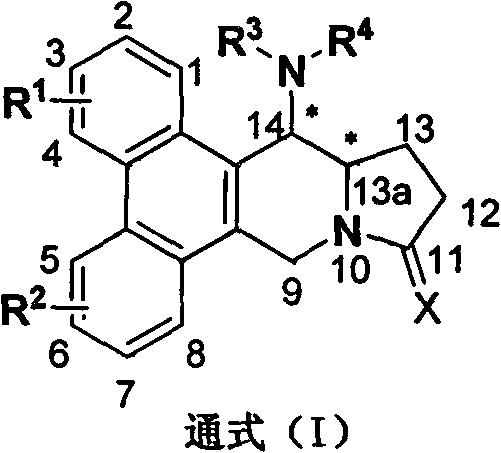
[0108] The chemical structural formula of preferred phenanthrene and indolizidine alkaloid C14 position amination derivative (I):
[0109]
[0110]
[0111] Compared with the known compounds, the above-mentioned preferred compounds have outstanding advantages, which are embodied in: (1) the light and thermal stability are significantly enhanced. Under the same conditions, they are irradiated with fluorescent lamps or controlled at 80°C for 24 hours and then qualitatively detected by NMR. No change occurred, while the control sample (R)-Antofine was mostly decomposed. (2) The water solubility is enhanced, the control sample (R)-Antofine is almost insoluble in water, while the preferred compound improves the water solubility. The above two points play a vital role in the application of compounds in pesticides.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com