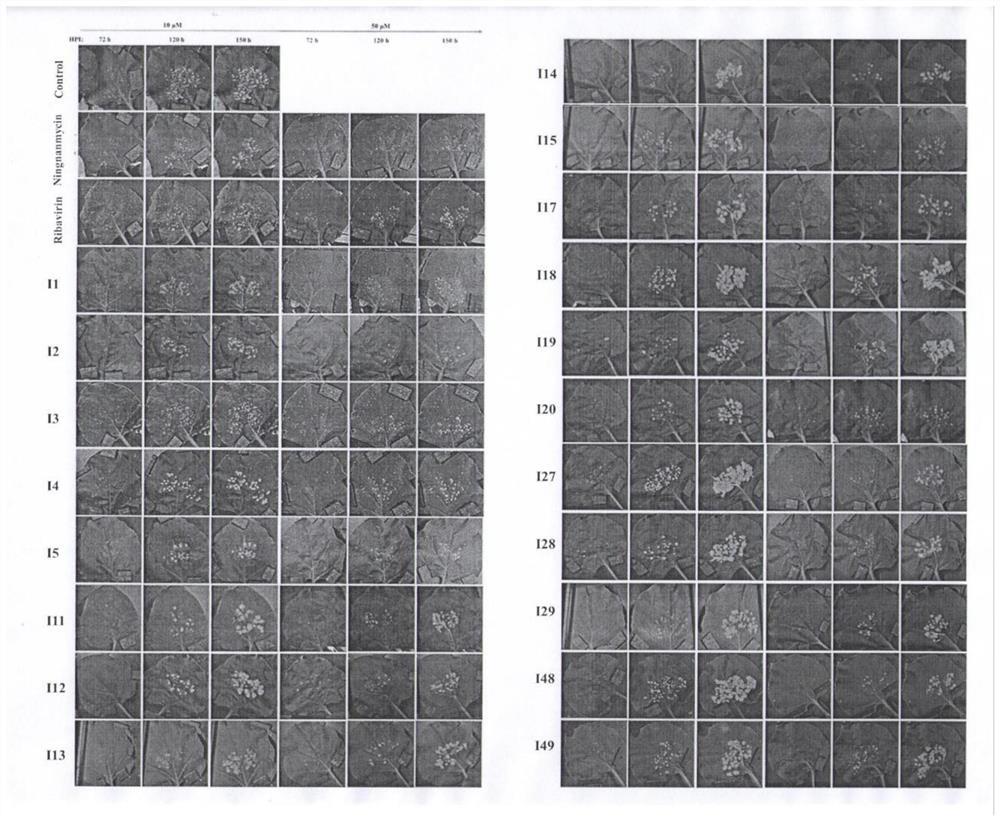
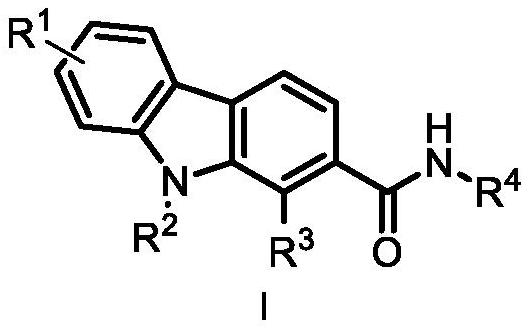
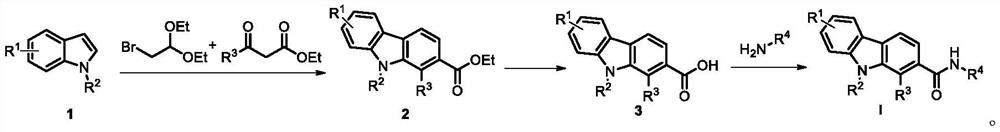
Carbazole alkaloid derivative and preparation method and application thereof
A technology for carbazole alkaloids and derivatives, which is applied in the field of carbazole alkaloid derivatives and their preparation, can solve the problems of difficulty in preventing and controlling plant virus diseases, lack of an immune metabolism system, affecting the yield and quality of tobacco leaves, and achieves good resistance to plants. The effect of virus activity, simple preparation method and obvious application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Numbering is the preparation of the compound of I2 in table 1, and its reaction path is:
[0031]
[0032] Its preparation method comprises the following steps:
[0033] 1) Dissolve indole (10mmol), bromoacetaldehyde diethyl acetal (10mmol), and ethyl acetoacetate (10mmol) in organic solvent ethanol (30mL), then add ZnCl 2 (1.0 mol), the above reaction solution was stirred and reacted at 60° C. for 2 h in a reactor equipped with magnetic stirring.
[0034] 2) Intermediate 2a (5.0 mmol) was dissolved in KOH (15.0 mmol) in EtOH / H 2 O (3:1) solution (20 mL), stirred at 70°C for 6 h, then acidified by adding dilute hydrochloric acid.
[0035] 3) Intermediate 3a (3 mmol) was dissolved in 15 mL of dry DMF, then HATU (3.3 mmol) and DIPEA (15 mmol) were added in sequence, and the reaction solution was reacted at room temperature for 8 h. The target compound I2 was obtained.
[0036] Spectral data of target compound I2: 1 H NMR (600MHz, DMSO-d 6 ,25℃)δ=11.26(s,1H),10.84...
Embodiment 2
[0038] Numbering is the preparation of the compound of I11 in table 1, and its reaction path is:
[0039]
[0040] Its preparation method comprises the following steps:
[0041] 1) Dissolve indole (10mmol), bromoacetaldehyde diethyl acetal (10mmol) and ethyl acetoacetate (10mmol) in organic solvent acetonitrile (30mL), then add AlCl 3 (1.0 mol), the above reaction solution was stirred and reacted at 50° C. for 2 hours in a reactor equipped with magnetic stirring.
[0042] 2) Intermediate 2a (5.0 mmol) was dissolved in 20 mL of ethanol, hydrazine hydrate (25 mmol) was added, and the above reaction solution was stirred and reacted at 80° C. for 6 hours in a reactor equipped with magnetic stirring.
[0043] 3) Intermediate 4a (2.0 mmol) and 3,4-dimethoxybenzaldehyde (2.0 mmol) were dissolved in ethanol (10 mL) respectively, and heated to reflux overnight to obtain the target compound I11.
[0044] Spectral data of target compound I11: 1 H NMR (600MHz, DMSO-d 6 ,25℃)δ=11.66...
Embodiment 3
[0046] Numbering is the preparation of the compound of I12 in table 1, and its reaction route is:
[0047]
[0048] Its preparation method comprises the following steps:
[0049] 1) Dissolve indole (10mmol), bromoacetaldehyde diethyl acetal (10mmol) and ethyl acetoacetate (10mmol) in the organic solvent toluene (30mL), then add HAc (0.5mL), and dissolve the above reaction solution in the In a reactor with magnetic stirring, the reaction was stirred at 60° C. for 4 h.
[0050] 2) Intermediate 2a (5.0 mmol) was dissolved in 20 mL of acetonitrile, hydrazine hydrate (30.0 mmol) was added, and the above reaction solution was reacted at 80° C. for 6 h in a reactor equipped with magnetic stirring.
[0051] 3) Intermediate 4a (2.0 mmol) and 3,4,5-trimethoxybenzaldehyde (2.0 mmol) were dissolved in ethanol (10 mL) respectively, and heated to reflux overnight to obtain the target compound I12.
[0052] Spectral data of target compound I12: 1 H NMR (600MHz, DMSO-d 6,25℃)δ=11.79(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com