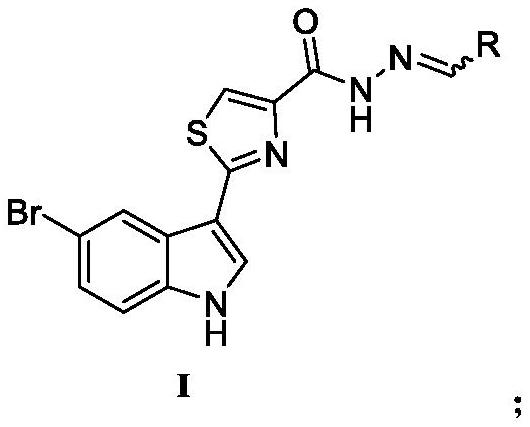
Camlexin derivative as well as preparation method and application thereof
A technology of derivatives and phytoalexin, applied in the field of fine chemicals, can solve the problem of low activity and achieve the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation method of described phytoalexin camalexin derivative, concrete steps are as shown in following reaction formula four:
[0037]
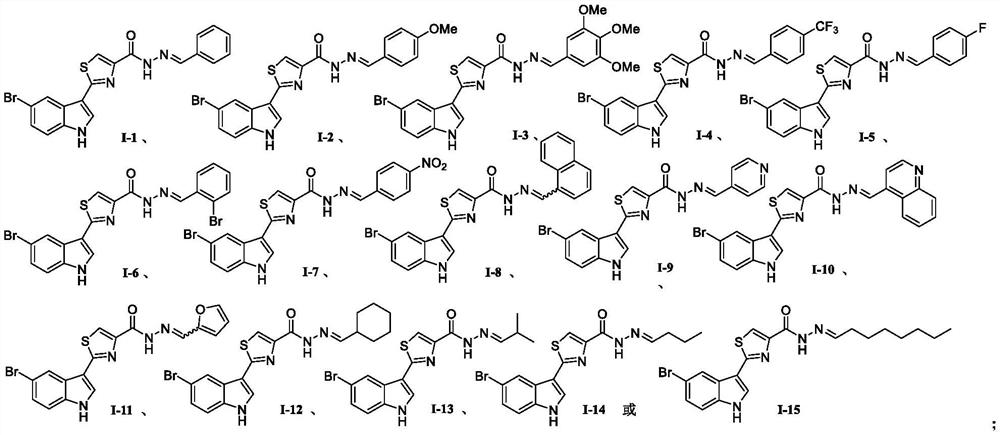
[0038] Using 5-bromo-1H-indole-3-carbothioamide shown in chemical structural formula 3e as raw material, reacting with ethyl 3-bromopyruvate in an ethanol solvent under heating and reflux to generate 2 as shown in chemical structural formula 5 -(5-bromo-1H-indol-3-yl)thiazole-4-formic acid ethyl ester intermediate product; the intermediate product is dissolved in ethanol, and 80% hydrazine hydrate is added to undergo hydrazinolysis reaction under heating conditions to obtain as chemical Important intermediate 2-(5-bromo-1H-indol 3-yl)thiazole-4-carbohydrazide shown in structural formula 6; finally 2-(5-bromo-1H-indol 3-yl)thiazole-4 -carbohydrazide reacts with different substituted aldehyde compounds to obtain 2-(5-bromo-1H-indol-3-yl)-N'-methylenethiazole-4 as shown in the general formula I - carbohydrazide.
[0039] The ...
Embodiment 1
[0041]
[0042] The preparation method of (E)-N'-benzylidene-2-(5-bromo-1H-indol-3-yl)thiazole-4-carbohydrazide shown in chemical structural formula I-1 is:
[0043] In the first step, chlorosulfonic acid isocyanate (12 mmol) was added dropwise to a solution of 5-bromoindole (10 mmol) in DMF (10 mL) under nitrogen protection at -50°C, and after the addition was completed, the temperature was raised to room temperature to continue the reaction for 1.5 hours. After the reaction was detected by TLC, the reaction system was poured into ice water, stirred for 30 minutes, and filtered with suction to obtain a yellow solid 5-bromo-1H-indole-3-carbonitrile with a yield of 99% and a melting point of 185-186°C; 1 H NMR (400MHz, CDCl 3 )δ9.18(s, 1H), 7.93(s, 1H), 7.76(s, 1H), 7.45(d, J=8.7Hz, 1H), 7.39(d, J=8.7Hz, 1H); 13 C NMR (100MHz, DMSO-d 6 )δ 135.9, 134.0, 128.4, 126.1, 120.7, 115.6, 115.0, 114.4, 84.0, the product was determined to be 5-bromo-1H-indole-3-carbonitrile.
[004...
Embodiment 2
[0049]
[0050] (E)-2-(5-bromo-1H-indol-3-yl)-N'-(4-methoxybenzylidene)thiazole-4-carbohydrazide shown in chemical structure I-2 The preparation method is:
[0051] Except that the fifth step is 4-methoxybenzaldehyde instead of benzaldehyde, the others are the same as in Example 1, a white solid with a yield of 56% and a melting point of >300°C; 1 H NMR (400MHz, DMSO-d 6 )δ12.08(s,1H),11.71(s,1H),8.59(s,1H),8.54(s,1H),8.30(s,1H),8.29(s,1H),7.72(d,J =8.7Hz, 2H), 7.49(d, J=8.6Hz, 1H), 7.39(d, J=8.6Hz, 1H), 7.06(d, J=8.7Hz, 2H), 3.83(s, 3H); 13 C NMR (100MHz, DMSO-d 6 C 20 h 16 BrN 4 o 2 S[M+H] + 455.0172, found (ESI + ) 455.0173, the product was determined to be (E)-2-(5-bromo-1H-indol-3-yl)-N'-(4-methoxybenzylidene)thiazole-4-carbohydrazide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com